- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Study confirms efficacy and safety of apremilast for treatment of plaques psoriasis
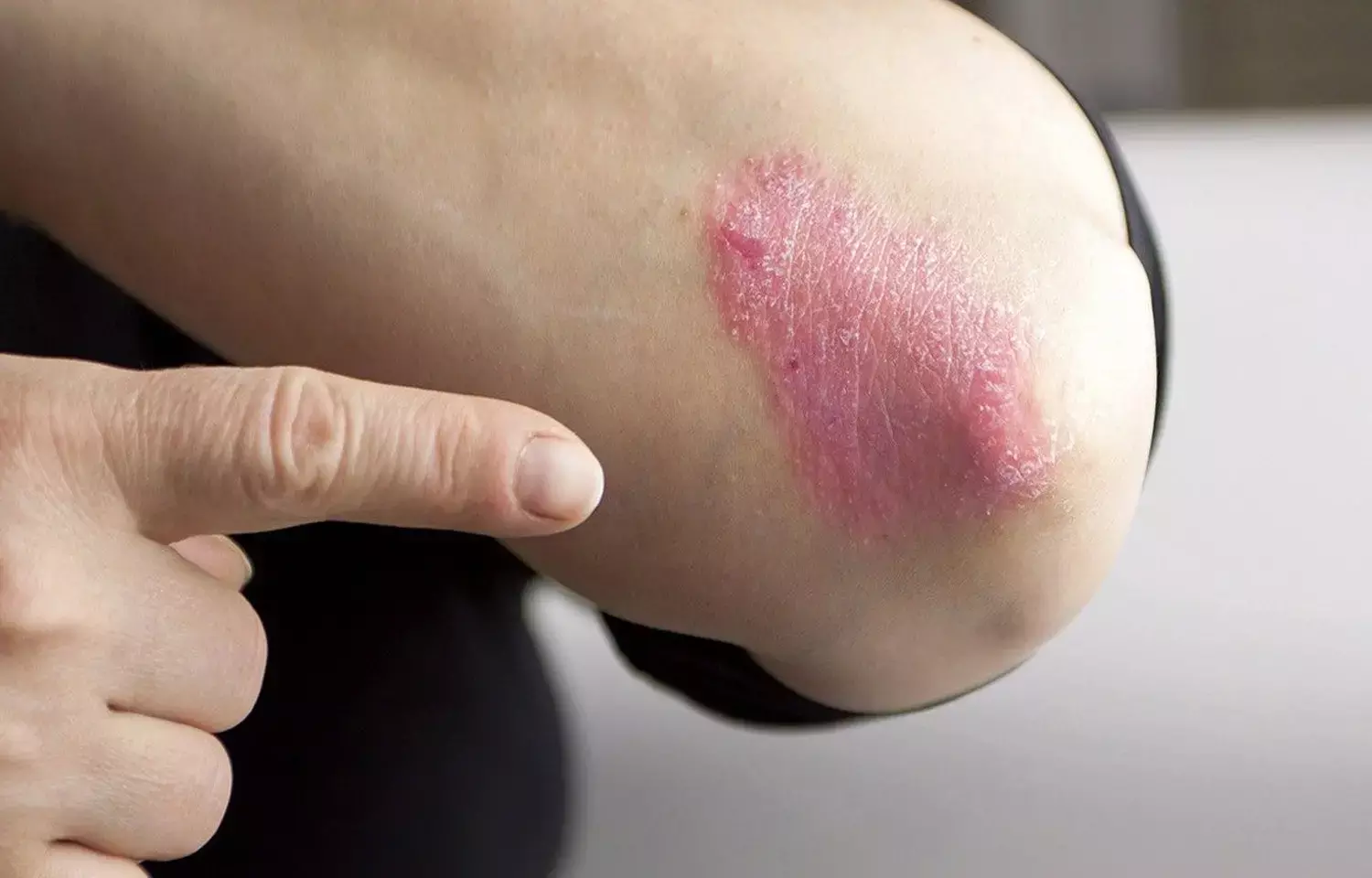
Italy: Apremilast in real-life experience confirmed the levels of safety and efficacy obtained in pivotal trials for the treatment of treat plaque psoriasis and adult psoriatic arthritis, according to a recent study published in the journal Dermatologic Therapy.
Specifically, the good initial response to the treatment predicts the maintenance or improvement of the outcome over 52 weeks. The efficacy is supported by an excellent safety profile even in frail patients.
Apremilast, a small molecule that has been approved to treat plaque psoriasis and adult psoriatic arthritis. Its short and long-term efficacy and safety have been demonstrated in pivotal studies, but there is still a scarcity of real-world data. Giulia Radi and the team aimed to make a report on the efficacy and safety of Apremilast in clinical practice in patients with moderate-to-severe plaque psoriasis, with a focus on therapeutic results obtained after 24 and 52 weeks of treatment.
From May to December 2018, 40 patients with plaque psoriasis were enrolled in the study. PASI, BSA PGA, and DLQI were administered at baseline 24 (W24) and 52 (W52) weeks after treatment began. The primary endpoint was to assess the proportion of patients who achieved PASI 75, PASI 90, and PASI 100 at weeks 24 and 52 of treatment. Another efficacy metric was the percentage of patients who achieved Minimal Disease Activity (MDA= PGA0/1 and DLQI 0/1) after 24 and 52 weeks of treatment. Researchers looked at the percentage of patients who achieved DLQI 0-1 at W24 and W52 as a secondary endpoint, as well as the long-term safety of Apremilast.
Only 33 of the 44 patients received the full evaluation. Of those, 5 patients (15%) reported a worsening of clinical condition with a decrease in PASI below PASI 50; 10 patients (30%) reached PASI 75, 14 patients (43%) reached PASI 90, and the remaining 4 patients (12%) reached PASI 100. In terms of the DLQI 0-1 endpoint, 24 patients out of 40 (60 percent) achieved the goal with a significant improvement in quality of life after 24 and 52 weeks of apremilast treatment. During the study, the average DLQI decreased from 10.2 to 1.5, with 60% of patients achieving DLQI 0-1. Adverse events were recorded in 28 patients (70%), including diarrhea (n14; 50%), nausea (n11; 39%), headache (n4; 14%), insomnia (n3; 11%), weight loss (n3; 11%), and cough (n4; 14%) (n2; 7%). The other 12 patients did not report any adverse events.
In conclusion, Apremilast in real-world use confirmed the efficacy and safety levels obtained in pivotal trials. The good initial response to the treatment, in particular, predicts the maintenance or improvement of the outcome over W52. The efficacy is backed up by an excellent safety profile, even in the frailest patients.
Reference:
Radi, G., Campanati, A., Diotallevi, F., Rizzetto, G., Martina, E., Bobyr, I., Giannoni, M., & Offidani, A. (2021). LONG‐TERM EFFICACY AND SAFETY OF APREMILAST IN THE TREATMENT OF PLAQUES PSORIASIS: A REAL‐WORLD , SINGLE‐CENTER EXPERIENCE. In Dermatologic Therapy. Wiley. https://doi.org/10.1111/dth.15179
Medical Dialogues consists of a team of passionate medical/scientific writers, led by doctors and healthcare researchers. Our team efforts to bring you updated and timely news about the important happenings of the medical and healthcare sector. Our editorial team can be reached at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


