- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Tildrakizumab Sustains Long-Term Efficacy for Scalp Psoriasis Treatment, reveals research
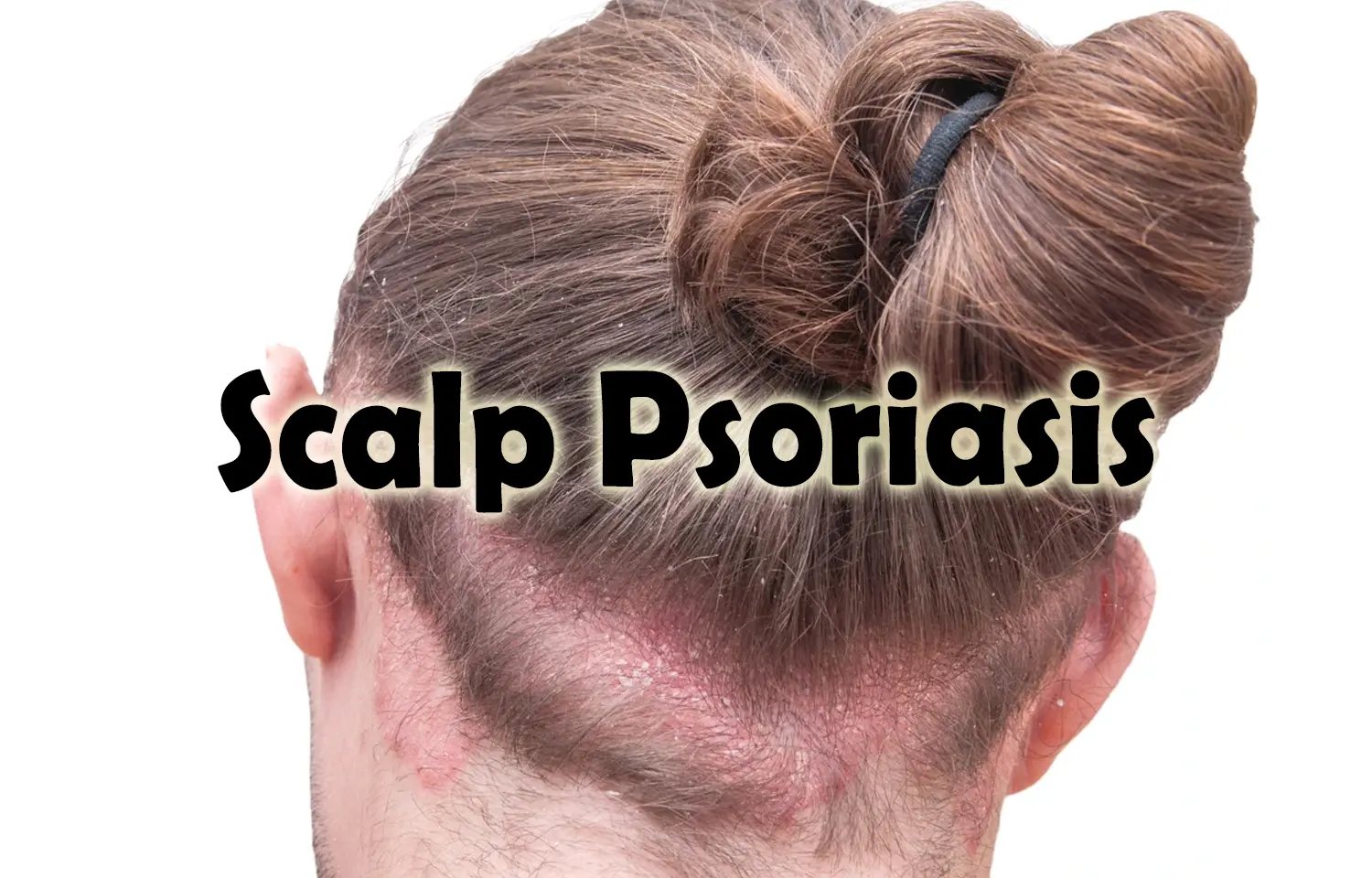
Researchers have established that tildrakizumab, an anti-interleukin-23 p19 antibody, can be used to maintain long-term efficacy and safety for moderate-to-severe scalp psoriasis. A recent study was conducted by Howard L. S. and colleagues which was published in the Journal of the American Academy of Dermatology.
Scalp psoriasis is one of the most difficult conditions to treat because of its visibility and impact on quality of life. Tildrakizumab is a targeted biologic therapy that acts on the inflammatory processes involved in this condition, and its use may offer hope for patients who do not respond to conventional therapies.
The study enrolled patients who were randomized to receive tildrakizumab (100 mg every 12 weeks) or placebo. At W16, the patients in the placebo group were switched to tildrakizumab for the rest of the trial. Efficacy endpoints were defined as achieving an IGA mod 2011; scalp score of 0 or 1 with a ≥2-grade improvement and ≥90% improvement in the PSSI 90 from baseline. The safety assessments were based on the incidence of adverse events.
Key Findings
W16 Primary Efficacy Results:
• Scalp IGA mod 2011 response rates: 49.4% for tildrakizumab compared with 7.3% for placebo.
• PSSI 90 response rates: 60.7% for tildrakizumab compared with 4.9% for placebo.
W52 Long-Term Efficacy:
• Scalp IGA mod 2011 response rates increased to 62.9% for tildrakizumab compared with 56.1% for placebo.
• PSSI 90 response rates achieved 65.2% for tildrakizumab compared with 57.3% for placebo.
Durability of Response:
• More than 80% of W16 responders to tildrakizumab were maintained at W52.
• No treatment-related serious adverse events were reported over the study period.
The study shows that in scalp psoriasis, the therapeutic efficacy of tildrakizumab has been maintained over a year of therapy. Tildrakizumab offers sustained efficacy and safety for the long-term treatment of scalp psoriasis. Therapy resulted in strong improvements in key efficacy measures, and the benefits lasted more than a year. These data position tildrakizumab as a promising option for the management of moderate-to-severe scalp psoriasis, an important area of unmet need in dermatological care.
Reference:
Sofen HL, Gebauer K, Spelman L, Yamauchi PS, Yao SL, Nishandar T, Kopeloff I, Crane M, Gogineni R, Kothekar M, Bagel J. Efficacy and Safety of Tildrakizumab for the Treatment of Moderate-to-Severe Plaque Psoriasis of the Scalp: Week 52 Results From a Phase 3b, Randomized, Double-Blind, Placebo-Controlled Trial. J Am Acad Dermatol. 2024 Dec 23:S0190-9622(24)03393-0. doi: 10.1016/j.jaad.2024.12.018
Dr Riya Dave has completed dentistry from Gujarat University in 2022. She is a dentist and accomplished medical and scientific writer known for her commitment to bridging the gap between clinical expertise and accessible healthcare information. She has been actively involved in writing blogs related to health and wellness.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


