- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Tofacitinib and ruxolitinib show efficacy in alopecia areata: shows meta-analysis
Source- Sacchidanand, Sarvajnamurthy & Srinivas, Sahana & Asha, G & Shilpa, Kanathur. Pattern of Pediatric Dermatoses at a Referral Centre. Indian journal of pediatrics.2012;81:10.1007/s12098-012-0904-8.
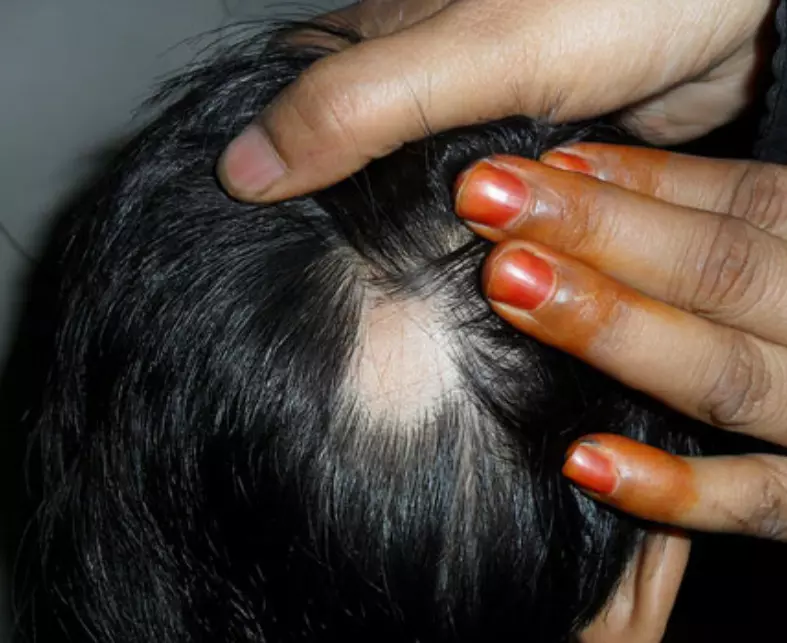
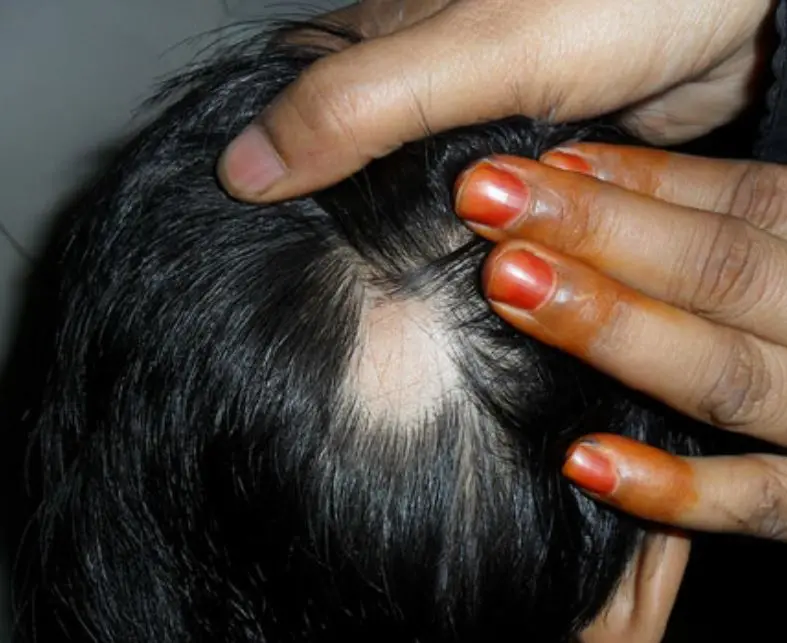
Tofacitinib and ruxolitinib show efficacy in alopecia areata: shows meta-analysis Alopecia areata is a common disorder of hair loss, treatment of which is challenging and often refractory to conventional therapy. Oral Janus kinase (JAK) inhibitors, including tofacitinib and ruxolitinib, have been used off-label to treat alopecia areata. As evidence for their use in alopecia areata is low a systemic review and meta-analysis of treatment outcomes of these two oral JAK-inhibitors in alopecia areata was published recently in the Indian Journal of Dermatology, Venereology and Leprology.
This was a systematic review and meta-analysis performed by searching for all articles published until January 28, 2019 on MEDLINE, Embase and Cochrane library. It focused on the Severity of Alopecia Tool50 (defined as the proportion of alopecia areata patients who achieved >50% hair regrowth from the baseline.) achievement rate, the frequency of adverse events and recurrence after discontinuation of treatment. A subgroup analysis was performed to evaluate the impact of study characteristics on the main outcome. In the subgroup analysis of adverse events, studies were divided according to the type of Janus kinase inhibitors and mean treatment duration.
Results
A total of 1244 studies were identified of which only 12 studies met the inclusion criteria. Of the 346 patients in these 12 studies, 288 had received oral tofacitinib and 58 had received oral ruxolitinib. Studies with oral baricitinib were excluded because they involved fewer than six patients.
The overall Severity of Alopecia Tool50 achievement rate was 66% (95% confidence interval, 54%–76%). The response rate to ruxolitinib was higher (79%, 95% confidence interval 66%– 87%) than tofacitinib (62%, 95% confidence interval 49%– 74%) but the difference was not statistically significant (P = .06).
Subgroup analysis revealed that drug choice, mean age, sex ratio and alopecia areata subtype ratio did not significantly affect the treatment response. Lower SALT50 achievement rates were noted with shorter treatment durations
Infections and laboratory abnormalities were the most common adverse events (98 and 65 cases of 319 patients, respectively). In infections upper respiratory infections, urinary tract infections, herpes zoster and herpes simplex infections were the most common. Patients treated for more than six months had a greater frequency of laboratory abnormalities as compared to those treated for shorter durations (24% vs. 7%; P = 0.04). Subgroup analyses did not reveal any significant differences in the frequency of adverse events between ruxolitinib and tofacitinib
Both the JAK inhibitors studied have different molecular targets – while tofacitinib is a pan- JAK inhibitor that strongly inhibits JAK-3, ruxolitinib inhibits both JAK-1 and JAK-2 to a similar extent. However, subgroup analysis did not show any significant differences in the treatment response or tolerability between tofacitinib and ruxolitinib. Recurrence of alopecia areata was observed within three months after discontinuation of treatment in the majority (74%) of patients.
To conclude this systematic review and meta-analysis of current evidence suggests that both oral tofacitinib and ruxolitinib are effective and well tolerated in the treatment of alopecia areata. Longer treatment durations > 6 months is more effective but at the increased risk of laboratory abnormalities. Clinicians should be aware of the expected efficacy, adverse events and high recurrence rate of oral JAK inhibitors for alopecia areata to closely monitor for adverse events and effectively counsel the patients before starting therapy.
Source- Yu DA, Kim YE, Kwon O, Park H. Treatment outcome of oral tofacitinib and ruxolitinib in patients with alopecia areata: A systematic review and meta-analysis. Indian J Dermatol Venereol Leprol 2021;87:621-7.
MBBS
Dr Manoj Kumar Nayak has completed his M.B.B.S. from the prestigious institute Bangalore medical college and research institute, Bengaluru. He completed his M.D. Dermatology from AIIMS Rishikesh. He is actively involved in the field of dermatology with special interests in vitiligo, immunobullous disorders, psoriasis and procedural dermatology. His continued interest in academics and recent developments serves as an inspiration to work with medical dialogues.He can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


