- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Topical Antiviral gel shows promise in managing molluscum contagiosum: Study
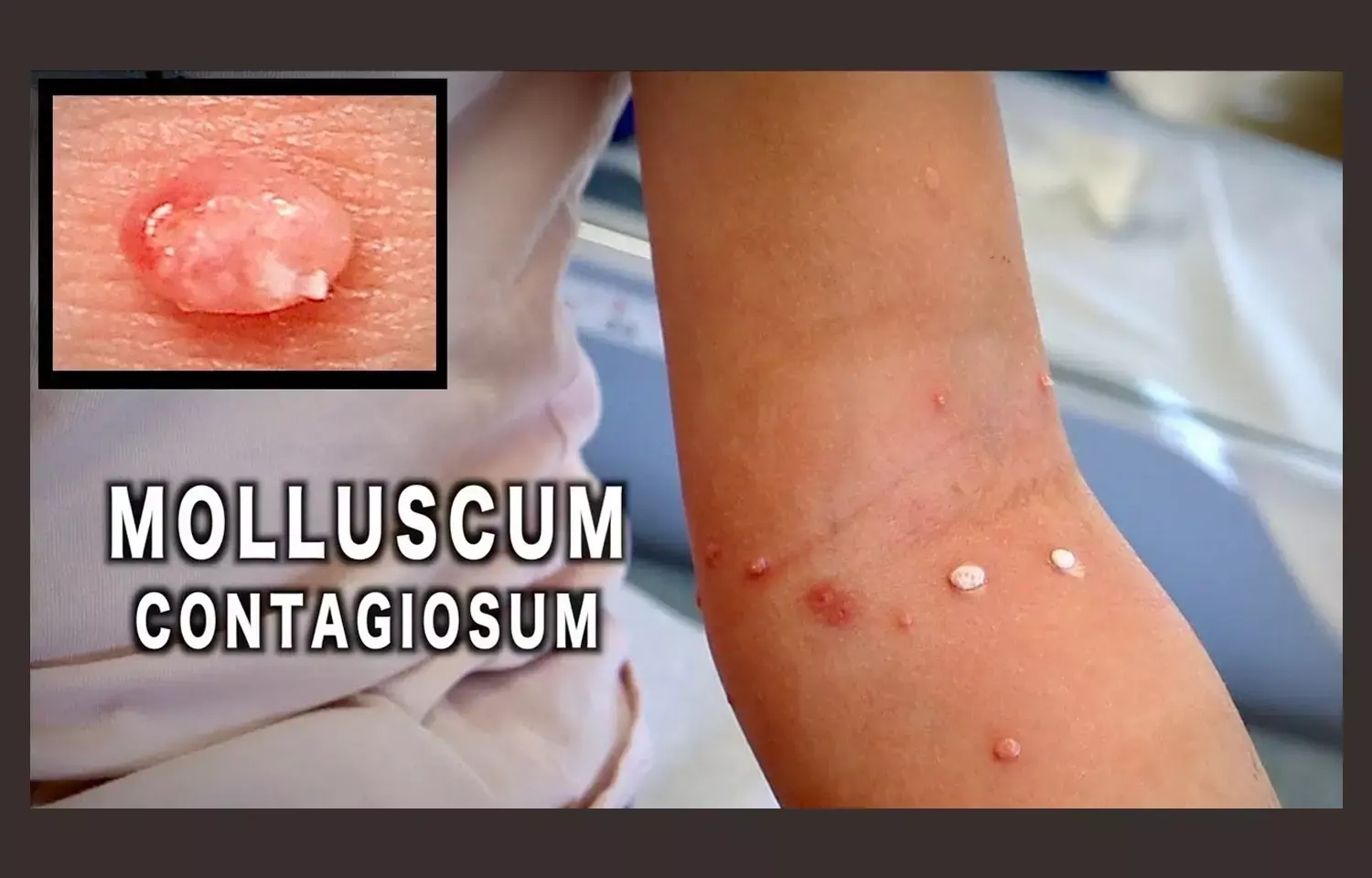
Molluscum is a common, contagious skin infection caused by the molluscipoxvirus, affecting approximately six million people in the U.S. annually, with the greatest incidence in children aged one to 14 years.
A recent press release by Novan, Inc. has highlighted that SB206, a topical antiviral gel, for the treatment of molluscum contagiosum, has shown positive efficacy and safety results in the B-SIMPLE4 pivotal Phase 3 clinical study.
B-SIMPLE4 is an ongoing multi-center, double-blind, randomized, vehicle-controlled study that exceeded its enrollment target by randomizing 891 patients (1:1 randomization) in the study, across 55 clinical sites. Patients were treated for up to 12 weeks with a follow-up visit at Week 24. The primary endpoint for the study is the proportion of patients with complete clearance of all treatable molluscum lesions at Week 12 (Intent-to-Treat or "ITT" population, where the analysis assumes that patients with missing data at Week 12 are considered treatment failures).
Results highlighted some important facts.
- Results demonstrated that SB206 met the primary endpoint, with 32.4% of patients achieving complete clearance of all lesions at week 12 compared with 19.7% of those treated with vehicle gel (P <.0001).
- Additionally, a greater proportion of SB2016-treated patients achieved a lesion count of 0 or 1 at week 12 (43.5% vs 24.6% with vehicle; P <.0001), 90% clearance of lesions at week 12 (43% vs 23.9% with vehicle; P <.0001), and complete clearance of all lesions at week 8 (19.6% vs 11.6% with vehicle; P =.0014).
- Consistent with results from the Company's Phase 2 and earlier Phase 3 studies, SB206 was found to be safe and well tolerated. No treatment-related serious adverse events ("TEAE") were reported.
There are currently no U.S. Food and Drug Administration ("FDA") approved therapies for the treatment of molluscum. The Company believes that SB206 as a topical, at-home, self or caregiver-applied therapy with a rapid treatment benefit, if approved, would satisfy an important patient-care need for the treatment of molluscum.
"The positive results from B-SIMPLE4 represent a transformational milestone for our employees, investors and most importantly, people living with molluscum. The strong safety and statistically significant efficacy results give us confidence as we move forward in preparing a New Drug Application to potentially bring SB206 to market and to patients in need of an effective therapy," commented Paula Brown Stafford, President and Chief Executive Officer of Novan. "We owe a great deal of gratitude to the collaborative efforts of our employees, partners, CROs, study investigators and participating patients who have contributed or participated in B-SIMPLE4."
For full article follow the link: Novan reports positive topline results from pivotal phase 3 trial of SB206 in patients with molluscum contagiosum. [press release]. Durham, NC: Novan, Inc.; June 11, 2021.
Source: Press release
Dr Satabdi Saha (BDS, MDS) is a practicing pediatric dentist with a keen interest in new medical researches and updates. She has completed her BDS from North Bengal Dental College ,Darjeeling. Then she went on to secure an ALL INDIA NEET PG rank and completed her MDS from the first dental college in the country – Dr R. Ahmed Dental College and Hospital. She is currently attached to The Marwari Relief Society Hospital as a consultant along with private practice of 2 years. She has published scientific papers in national and international journals. Her strong passion of sharing knowledge with the medical fraternity has motivated her to be a part of Medical Dialogues.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


