- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Tralokinumab Shows Sustained Efficacy in Hand Atopic Dermatitis: Study
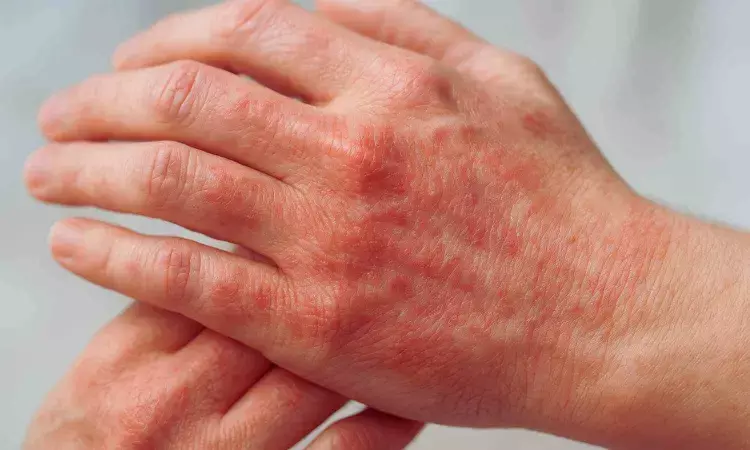
The ADHAND trial has demonstrated that tralokinumab significantly improved skin clarity, itch, and pain in adults with moderate-to-severe hand atopic dermatitis. All primary and secondary endpoints were met at 16 and 32 weeks, highlighting its long-term effectiveness for patients requiring systemic therapy.
ADHAND is a phase 3b, interventional, adaptive, placebo-controlled clinical trial evaluating the efficacy and safety of tralokinumab 300 mg administered every two weeks as a monotherapy compared with placebo in patients living with moderate-to-severe atopic dermatitis on the hands who are candidates for systemic therapy.1 After the first 16 weeks double-blinded treatment period, all patients received open-label, 300 mg tralokinumab every 2 weeks for 16 weeks.
The trial met all key primary and secondary endpoints at Week 16 as well as all endpoints at Week 32, including efficacy on extent and severity of disease, itch, pain, sleep, and health-related quality of life and the safety and tolerability of continued tralokinumab treatment.
“We are truly excited about these 32-week key results, which bring new hope for patients suffering from atopic dermatitis with moderate-to-severe hand involvement,” said Christophe Bourdon, Chief Executive Officer at LEO Pharma. “These results build on the impressive 16-week data, reaffirming our confidence in how tralokinumab can make a difference for patients living with this debilitating disease. Hand involvement is not only physically painful and functionally limiting, but it can also be emotionally burdensome for patients. Seeing such positive outcomes in these visible, high burden areas gives us a brighter outlook on what we can achieve for those living with this challenging condition.”
Detailed results of this Week 32 analysis will be submitted for scientific presentation and publication at a later date.
Positive 16-week data presented at ISAD 2025
Tralokinumab showed statistically significant improvement compared to placebo in all primary and key secondary endpoints such as clear or almost clear skin, itch and pain at Week 16 in ADHAND.
“The hands are a hard-to-treat area and atopic dermatitis with hand involvement is often very burdensome for patients to live with. While the genitals and head-and-neck are also high burden areas, the hands are particularly susceptible to external triggers, which can make disease on the hands especially challenging to manage,” says Teodora Festini, Global Medical Affairs at LEO Pharma and presenting author at ISAD Congress 2025 in Melbourne, Australia. “The detailed 16-week results and now these high-level findings from week 32 underscores LEO Pharma's commitment to making a difference for patients with atopic dermatitis - particularly where their disease is hard to treat.”
The data showed an early onset of effect observed from week 2 in the primary endpoint. There was a statistically significant difference in the proportion of patients who achieved an Investigator’s Global Assessment for Atopic Hand Eczema (IGA-AHE) score of 0/1 – clear or almost clear skin on the hands at Week 16 (40.0% of patients (N=156) compared with 10.6% for placebo (N=79)).
At Week 16, tralokinumab also achieved a statistically significant reduction in both itch and pain versus placebo; 47.3% of tralokinumab treated patients achieved a ≥4point reduction in HESD itch versus 20.7% with placebo, and 45.3% achieved a ≥4point reduction in HESD pain versus 13.3% with placebo, indicating meaningful improvement in these key symptoms.
Furthermore, 41.7% of tralokinumab treated patients achieved HECSI90 versus 10.9% with placebo, a difference of 30.8 percentage points, indicating a substantial and clinically meaningful improvement at Week 16.
Tralokinumab was generally well tolerated with no new safety signals identified, continuing the known overall safety profile of the treatment. The majority of adverse events observed were non-serious, mild or moderate in severity – and the number of adverse events is consistent between tralokinumab (60.3%) and placebo (60.8%). Similarly, when considering adverse events of special interest, the number of patients suffering from conjunctivitis is on par between the tralokinumab arm (3.8%) and the placebo arm (3.8%).
The positive Week 16 data was presented at the 15th Georg Rajka International Symposium on Atopic Dermatitis (ISAD), held from October 24 – 26, 2025, in Melbourne, Australia.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


