- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Triple-Combination Acne Treatment Shows Promise in Clinical Studies
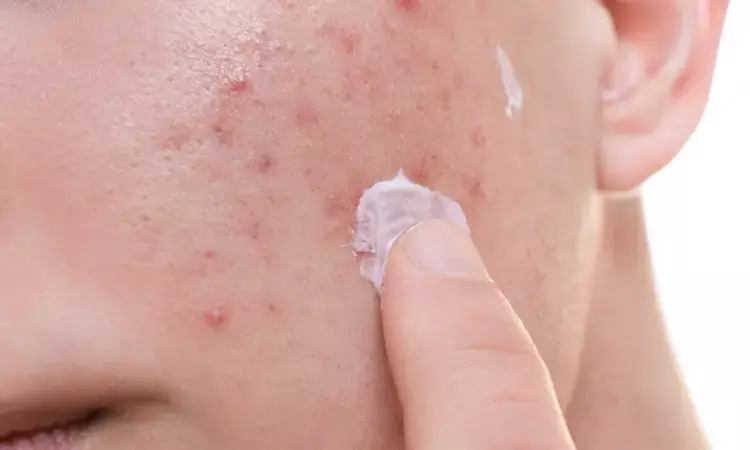
A triple-pronged approach to acne treatment has shown remarkable promise, outshining conventional single or double treatments in a recent study published in the Journal of the American Academy of Dermatology. The treatment in question, a topical gel known as IDP-126, combines an antibiotic, antibacterial agent, and a retinoid.
The IDP-126 gel, which contains a combination of clindamycin phosphate, adapalene, and benzoyl peroxide, has undergone rigorous testing in two phase 3 clinical trials. The aim was to assess its effectiveness, safety, and tolerability in treating acne. These trials involved a total of 363 participants aged nine years and older who were suffering from moderate-to-severe acne.
Participants were randomly divided into two groups: one received daily applications of IDP-126, while the other used a vehicle gel as a control. The co-primary endpoints of the trials were twofold: firstly, whether participants achieved a reduction of at least two grades from their baseline Evaluator's Global Severity Score (EGSS) and achieved clear or almost clear skin (termed "treatment success"), and secondly, the change from the baseline in the counts of inflammatory and noninflammatory lesions. The trials also closely monitored any treatment-emergent adverse events.
By the end of the 12-week trials, nearly half of the participants (around 50%) who used IDP-126 had achieved treatment success. This was a stark contrast to the control group, where only around a quarter of participants saw similar success. These results were statistically significant.
Furthermore, the IDP-126 group also showed a substantial reduction in both inflammatory and noninflammatory lesions compared to the control group. This indicates the potential of the triple-combination gel in effectively addressing various acne-related issues.
Notably, most of the treatment-emergent adverse events reported in both groups were of mild to moderate severity, suggesting that the IDP-126 gel is well-tolerated.
While these results are promising, it's essential to consider the limitations of the trials, including potential biases in assessing the severity of acne, the relatively short duration of treatment, and the specific demographics of the study participants.
Reference:
Stein Gold, L., Lain, E., Del Rosso, J. Q., Gold, M., Draelos, Z. D., Eichenfield, L. F., Sadick, N., Werschler, W. P., Gooderham, M. J., & Lupo, M. (2023). Clindamycin phosphate 1.2%/adapalene 0.15%/benzoyl peroxide 3.1% gel for moderate-to-severe acne: Efficacy and safety results from two randomized phase 3 trials. In Journal of the American Academy of Dermatology (Vol. 89, Issue 5, pp. 927–935). Elsevier BV. https://doi.org/10.1016/j.jaad.2022.08.069
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


