- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
180-day implantable CGM found safe and effective for managing blood sugar: Study
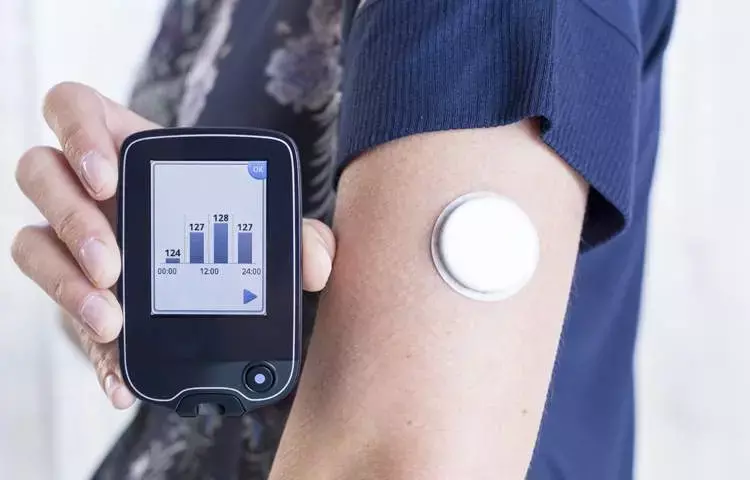
A 180-day implantable continuous glucose monitor was found to be safe and effective for use among diabetic patients.
A 180-day implantable continuous glucose monitor (CGM) was found to be both safe and effective, according to a Prospective Multi-Centre Imaging Study for Evaluation (PROMISE) conducted by a research team led by Satish K. Garg, MD, of the University of Colorado in Aurora.
Diabetes has emerged as a major global health concern over the past few decades. It is a chronic condition that affects the way the body processes blood sugar.
Diabetic patients either don't produce enough insulin, or the body resists insulin. In such patients, regular monitoring of glucose levels is essential. Keeping this in mind Garg's research team conducted the PROMISE study across eight clinical research sites, specifically testing two different sensors used with the CGM system. The study was put forth during the virtual American Diabetes Association (ADA) Scientific Sessions.
It included 181 participants, with an average age of 49. The cohort was split between men and women and more than 90% of the cohort was white. The average duration of diabetes was 22 years. Average baseline HbA1c was 7.6%, and 70% had type 1 diabetes.
The participants had a primary sensor implanted into one arm; one half had an identical sensor placed into the other arm, while the other half had a different sensor placed into the second arm -- a Sacrificial Boronic Acid (SBA) sensor -- which has a specific chemical makeup aimed at reducing oxidation of the glucose-binding indicator chemistry.
Participants were seen at 10 clinic visits that took place during the 180 days of use, each visit lasting up to 10 hours. At these visits, participants underwent hyperglycemic and hypoglycemic challenges to test the full 40-400 mg/dL blood glucose range. CGM readings were compared with a reference glucose reading from a Yellow Springs Instruments (YSI) 2300 glucose analyzer.
Garg and team reported the following findings:
- Average HbA1c improved across the cohort, dropping from 7.6% to 7.3% at day 180.
- only 65% of the primary sensors survived up to the full 180 days of implantation. Nearly all survived to day 90 (98%), 90% were still working by day 120, and only 74% survived to day 150.
- The SBA sensor performed far better, as 90% lasted the entire 180-day wear
- They also were slightly more accurate in regard to hypoglycemia, with a 7.5% and 7.7% mean absolute difference at ranges of 40-60 mg/dL and 61-80 mg/dL
- The SBA sensors also performed better in alerting the patient to hypoglycemia (glucose of 60 mg/dL), with a true alert rate of 73% versus 68% with the primary sensor.
"These CGM are accurate, safe for use up till 180 days and show no serious adverse events with respect to insertion or removal of the devices," the team concluded.
Garg additionally remarked, "Clearly, this biochemical change that has been made in the SBA sensor is probably the future way to go. The majority of the patients can have 6 months of data available to them with one particular implant."
Primary Source
American Diabetes Association
Source Reference: Garg SK, et al "Evaluation of the next generation 180-day long-term implantable Eversense CGM system: PROMISE study" ADA 2021; Abstract 149-OR.
Medical Dialogues Bureau consists of a team of passionate medical/scientific writers, led by doctors and healthcare researchers. Our team efforts to bring you updated and timely news about the important happenings of the medical and healthcare sector. Our editorial team can be reached at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


