- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Exhalation delivery system reduces inflammation of nasal cavity and symptoms of chronic sinusitis
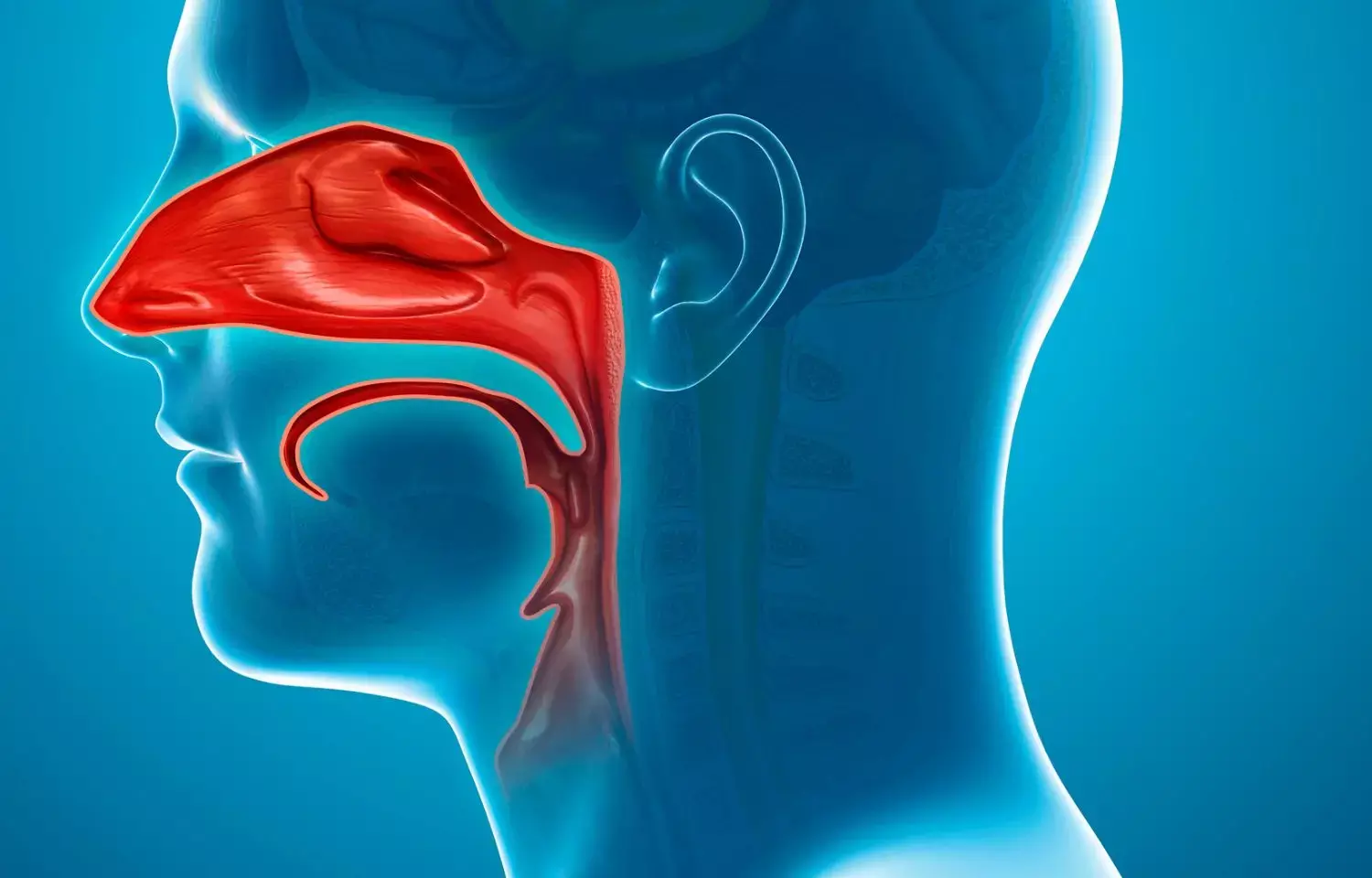
An exhalation delivery system that uses fluticasone propionate nasal spray may reduce symptoms of chronic sinusitis and local inflammation of the nasal cavity and adjacent sinus cavities, according to topline study results announced by OptiNose
The study reveals the statistically significant benefits of XHANCE in the ReOpen2 trial for both the symptoms co-primary endpoint and the CT scan co-primary endpoint. Significant improvement was demonstrated in patients with chronic sinusitis who did not have nasal polyps treated with both doses of XHANCE® (fluticasone propionate) nasal spray with the Exhalation Delivery System™ in the ReOpen2 clinical trial compared to patients receiving a vehicle Exhalation Delivery System (placebo).
The co-primary endpoints were a patient-reported composite symptom score (comprising nasal congestion, facial pain or pressure, and nasal discharge) measured at week 4 and an objective measure of disease in the sinus cavities at week 24 (measured by average of the percentages of opacified volume on CT scan across the ethmoid and maxillary sinuses).
"ReOpen2 is a large, international, controlled trial, studying 222 patients with chronic sinusitis who did not also have nasal polyps. Currently there are no FDA-approved drug treatments for this large patient population," said Ramy Mahmoud, MD, MPH, President of Optinose. He went on to say, "With top-line results showing that patients with chronic sinusitis experienced significant improvement in both symptoms and inflammation inside the sinuses, ReOpen2 confirms and builds on the positive results from ReOpen1 and, importantly, provides evidence supporting the effectiveness of XHANCE in the very large chronic sinusitis population without nasal polyps.
This program provides the first-ever body of controlled trial evidence we are aware of for a nasal medication to produce both improvement in symptoms and reduction of inflammation in the sinus cavities for patients suffering from chronic sinusitis regardless of the presence of nasal polyps.
We believe these results are important to the tens of millions of people suffering from chronic sinus disease, so our team is working to quickly complete the analyses of both ReOpen1 and ReOpen2 and have begun the work necessary to seek a new indication that expands access to XHANCE for this broader group of patients. We would like to express our gratitude to the healthcare professionals at our research sites and, most especially, to all the patients with chronic sinusitis whose altruistic participation made it possible to offer new hope to others who suffer from the same symptoms."
"I see patients every day who suffer greatly from the symptoms of chronic sinusitis, despite availability of current nasal treatments. I'm excited to see this important confirmatory data showing the benefits of XHANCE in this challenging population. This evidence has potential to change the treatment paradigm for chronic sinusitis patients." said Rick Chandra, M.D., professor of otolaryngology, chief of rhinology, sinus & skull base surgery, Vanderbilt University.
"The potential addition of the first FDA-approved medication to be an option for chronic sinusitis patients with or without nasal polyps also fits nicely in a stepwise treatment paradigm for those who continue to have symptoms despite trying standard nasal sprays, before they escalate to surgery or other treatments."
The safety profile and tolerability of XHANCE in this trial were generally consistent with its currently labelled safety profile. Adverse events occurring at a rate of more than 3% with XHANCE and more common than the Exhalation Delivery System placebo group in this trial were: epistaxis, COVID-19, headache, and depression.
When pre-planned analyses are completed, detailed results from ReOpen2 will be submitted for publication in a peer-reviewed journal and for presentation at future medical meetings.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


