- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Indian-origin boy denied Duchenne muscular dystrophy treatment in UK
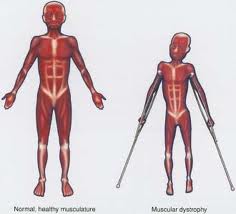
London, Oct 19: Six children, including a 6-year-old Indian-origin boy, have been denied life-saving treatment from a rare disease due to high costs by UK's state-funded health service.
Kirath Mann suffers from Duchenne muscular dystrophy (DMD), which affects one in 3,500 boys in Britain and leaves many in a wheelchair before their 10th birthday.
His family, from Coventry in the West Midlands region of England, joined forces with other families to campaign to end delays to a breakthrough treatment called Translarna but failed due to high expenses involved.
"We are devastated at having to face yet more disappointment after another hurdle has been put in our way.
"All these organisations and National Health Service (NHS) bodies that have been involved in the decision do not seem to be appreciative that this is a rare disease and very progressive one as well," Kirath's mother Jaspal told 'The Coventry Telegraph'.
Kirath and five other boys with the condition even wrote personal letters to British Prime Minister David Cameron in June in a bid to get the drug that could save their lives.
The National Institute for Health and Care Excellence (NICE), which decides on commissioning new drugs for the NHS, has decided that drug manufacturers PCT Therapeutics need to present more information on the benefits of the drug to coincide with the cost as Translana can cost up to 4,00,000 pounds per patient a year.
"It was at NICE's discretion to give a positive decision and it was in their remit to do that. But they decided to go for a different approach," she added.
The drug - also known as Ataluren - is the first-ever to tackle the causes of DMD and was approved in Europe in August last year and is available in France, Spain, Germany, Italy and Denmark, but not in the UK.
"We are disappointed not to be able to recommend Ataluren in this draft guidance. After considering the evidence, and the opinions of the clinical and patient experts, the Committee agreed that Ataluren represents an important development in the treatment of DMD.
"It could potentially prolong the time before children have to use a wheelchair, compared with best supportive care," said Prof Carole Longson, NICE health technology evaluation centre director. From Aditi Khanna
Kirath Mann suffers from Duchenne muscular dystrophy (DMD), which affects one in 3,500 boys in Britain and leaves many in a wheelchair before their 10th birthday.
His family, from Coventry in the West Midlands region of England, joined forces with other families to campaign to end delays to a breakthrough treatment called Translarna but failed due to high expenses involved.
"We are devastated at having to face yet more disappointment after another hurdle has been put in our way.
"All these organisations and National Health Service (NHS) bodies that have been involved in the decision do not seem to be appreciative that this is a rare disease and very progressive one as well," Kirath's mother Jaspal told 'The Coventry Telegraph'.
Kirath and five other boys with the condition even wrote personal letters to British Prime Minister David Cameron in June in a bid to get the drug that could save their lives.
The National Institute for Health and Care Excellence (NICE), which decides on commissioning new drugs for the NHS, has decided that drug manufacturers PCT Therapeutics need to present more information on the benefits of the drug to coincide with the cost as Translana can cost up to 4,00,000 pounds per patient a year.
"It was at NICE's discretion to give a positive decision and it was in their remit to do that. But they decided to go for a different approach," she added.
The drug - also known as Ataluren - is the first-ever to tackle the causes of DMD and was approved in Europe in August last year and is available in France, Spain, Germany, Italy and Denmark, but not in the UK.
"We are disappointed not to be able to recommend Ataluren in this draft guidance. After considering the evidence, and the opinions of the clinical and patient experts, the Committee agreed that Ataluren represents an important development in the treatment of DMD.
"It could potentially prolong the time before children have to use a wheelchair, compared with best supportive care," said Prof Carole Longson, NICE health technology evaluation centre director. From Aditi Khanna
Meghna A Singhania is the founder and Editor-in-Chief at Medical Dialogues. An Economics graduate from Delhi University and a post graduate from London School of Economics and Political Science, her key research interest lies in health economics, and policy making in health and medical sector in the country. She is a member of the Association of Healthcare Journalists. She can be contacted at meghna@medicaldialogues.in. Contact no. 011-43720751
Next Story


