- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Dupilumab notably reduces hives and hitch in patients with chronic spontaneous urticaria: Study
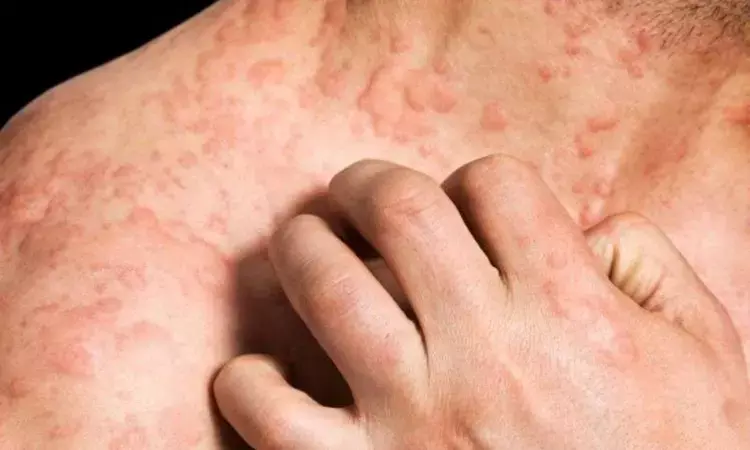
USA: Results from a phase 3 trial revealed that dupilumab is well-tolerated and causes significant improvement in patients with H1 antihistamine-resistant chronic spontaneous urticaria (CSU). The findings of the trial were presented at the annual meeting of the American Academy of Allergy, Asthma & Immunology and subsequently published in the Journal of Allergy and Clinical Immunology.
Chronic spontaneous urticaria (CSU) causes recurrent itchy hives and/or angioedema and significantly impacts the quality of life. Many patients experience a substantial disease burden despite treatment with recommended and escalated doses of H1 antihistamines. Considering this, Marcus Maurer, Charite-Universitatsmedizin Berlin, and colleagues aimed to evaluate the safety and efficacy of dupilumab in patients aged d >_6 years with CSU who remained symptomatic despite treatment with H1 antihistamines in LIBERTY-CSU CUPID Study A -- a randomized, placebo-controlled, 24-week, phase 3 trial.
The trial included patients on a standard or <_ 4-fold dose of antihistamines. They were randomized to receive add-on dupilumab (n 5 70) 300 mg (adults/adolescents >_ 60 kg) or 200 mg (adolescents < 60 kg/children >_ 30 kg) or matching placebo (n 5 68) subcutaneously every 2 weeks. change from baseline at Week 24 in Itch Severity Score over 7 days (ISS7) was the primary endpoint. Key secondary endpoints included Urticaria Activity Score over 7 days (UAS7).
Key findings of the study include:
- Baseline characteristics were generally balanced across treatment groups.
- Mean ISS7 and UAS7 (dupilumab/placebo) at baseline were 16.1/15.7 and 31.9/30.8, respectively.
- At Week 24, least squares (LS) mean change in ISS7 (range:0-21) from baseline was -10.2/-6.0 for dupilumab/placebo, respectively (LS mean difference -4.2, P50.0005) and for UAS7 (range:0-42) was -20.5/-12.0 (difference -8.5).
- Overall treatment-emergent adverse events (TEAEs) were comparable between dupilumab and placebo: 35 (50.0%)/40 (58.8%); occurrence of injection site reactions was 8 (11.4%)/9 (13.2%), conjunctivitis 0/1 (1.5%), and serious TEAEs 2 (2.9%)/5 (7.4%).
The researchers conclude, "dupilumab demonstrated clinically meaningful and statistically significant improvements in patients with H1 antihistamine- resistant CSU and was well tolerated."
Reference:
The study titled, "Dupilumab Significantly Reduces Itch and Hives in Patients With Chronic Spontaneous Urticaria: Results From a Phase 3 Trial (LIBERTY-CSU CUPID Study A)," was published in the Journal of Allergy and Clinical Immunology.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


