- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Handheld ECG for Measurement of QTc interval Receives FDA Clearance
AliveCor's KardiaMobile 6L device which can calculate patients' QTc interval recently has received FDA clearance.
The US Food and Drug Administration has given clearance to KardiaMobile 6L device for calculating patients' QTc interrval by healthcare professionals.
AliveCor, a medical device and artificial intelligence company that sells ECG hardware and software for consumer mobile devices has recently received the clearance .
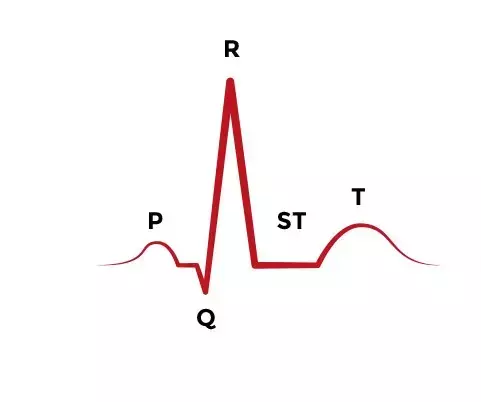
The QT interval on an electocardiogram is the time from the beginning of the QRS complex, representing ventricular depolarization, to the end of the T wave, resulting from ventricular repolarization. Due to the effects of heart rate, the corrected QT interval (QTc) is frequently used.
These developments will enable healthcare professionals to use the KardiaMobile 6L device to obtain an ECG which they can use to manually measure their patients' QT interval. AliveCor claims that this device is fast, easy, and convenient as it can be done by the healthcare professional in the office and even remotely by the patient. KardiaMobile 6L is the first and only hand-held ECG device that is FDA cleared for measurement of QTc.
QT prolongation can stem from congenital long QT syndrome, many disease states or electrolyte abnormalities. Patients with a prolonged QTc are at greater risk for their hearts to go into a potentially dangerous arrhythmia. QT prolongation can also be a potential side effect of more than 100 FDA-approved medications including certain antiarrhythmic medicines, cancer therapies, antifungals, antipsychotics, antidepressants, antibiotics, multiple sclerosis (MS) medications, and opioids etc.
Medicines known to cause QT prolongation are commonly prescribed by healthcare professionals, who may not have ECG capabilities readily available. Some physicians view QT prolongation as a barrier to prescribing these potentially life-saving medicines.
"Patient safety is paramount, and this is why we are proud to offer physicians the ability to monitor QTc through the convenience and quality of our device. It is our hope that this important FDA clearance will help healthcare professionals identify and save patients from this potentially life-threatening condition," Priya Albani, CEO of AliveCor told mediapersons.
Dr Kartikeya Kohli is an Internal Medicine Consultant at Sitaram Bhartia Hospital in Delhi with super speciality training in Nephrology. He has worked with various eminent hospitals like Indraprastha Apollo Hospital, Sir Gangaram Hospital. He holds an MBBS from Kasturba Medical College Manipal, DNB Internal Medicine, Post Graduate Diploma in Clinical Research and Business Development, Fellow DNB Nephrology, MRCP and ECFMG Certification. He has been closely associated with India Medical Association South Delhi Branch and Delhi Medical Association and has been organising continuing medical education programs on their behalf from time to time. Further he has been contributing medical articles for their newsletters as well. He is also associated with electronic media and TV for conduction and presentation of health programs. He has been associated with Medical Dialogues for last 3 years and contributing articles on regular basis.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


