- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Oral Milvexian effective in prevention of venous thromboembolism: NEJM
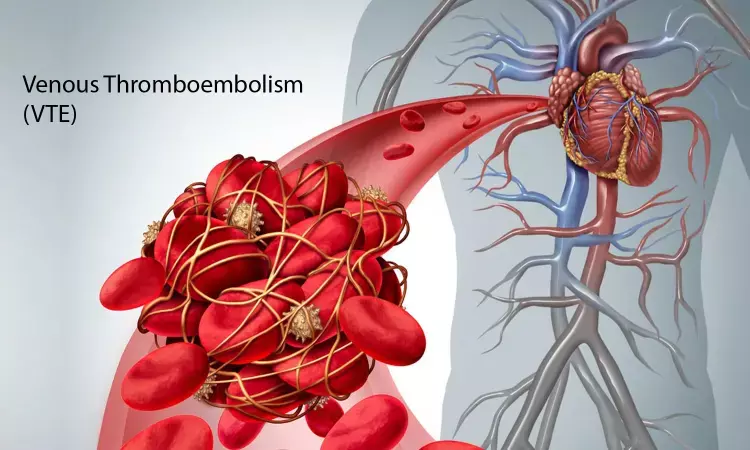
Hamilton, ON - Milvexian has been found to be effective for prevention of venous thromboembolism and it lowers risk of bleeding in patients undergoing knee arthroplasty, finds a new study. The drug achieves this effect by Postoperative factor XIa inhibition and it can be used with minimal side effects.find researchers at McMaster University.
Milvexian is unique in that it works by targeting factor XIa, a clotting enzyme that causes dangerous thrombosis, blood clots, but is not vital for stopping bleeding from injuries.
Researchers compared milvexian with enoxaparin for prevention of blood clots in 1,242 patients from18 countries undergoing knee replacement surgery who were enrolled between June 2019 and February 2021.
They found that at a total daily dose of 100 mg or more, milvexian resulted in better clot protection but no increase in bleeding compared with enoxaparin, the control drug. Milvexian was evaluated in daily doses ranging from 25 to 400 mg; there was no increase in bleeding over this wide range of doses.
"The major side effect of current oral anti-clotting drugs is bleeding, and the fear of bleeding leads to their underuse. This sets the need for safer oral anticoagulants and that is where milvexian comes in," said senior author Jeffrey Weitz.
He is a professor of medicine and biochemistry and biomedical sciences at McMaster. He is also the executive director of the Thrombosis and Atherosclerosis Research Institute of McMaster and Hamilton Health Sciences.
"Blood clots are responsible for 1 in 4 deaths worldwide. Anticoagulants (blood thinners) are a mainstay for the treatment and prevention of clots in veins and arteries and we urgently need safer oral medications to reduce the burden from what are often lifelong conditions."
The study was published today in the New England Journal of Medicine and Weitz presented a summary of the findings at a late breaking session at the 2021 American Heart Association Scientific Sessions.
Weitz said that blood clots are the underlying cause of heart attack, stroke, deep vein thrombosis, and pulmonary embolism. Many of these conditions require lifelong anticoagulant treatment. Therefore, there is a need for safer oral anticoagulants like milvexian.
He said the study focused on patients undergoing knee replacement surgery because they are at high risk for postoperative blood clots and such clots can be readily identified with venograms, x-rays of the veins of the legs. Therefore, this patient population provides an ideal testing ground for new anticoagulants because effective and safe doses can be identified.
Weitz said his milvexian study is the first of several investigations of oral factor XIa inhibitors. The results of the other studies will likely be released next year.
Funding for the study was provided by the pharmaceutical firms Bristol Myers Squibb and Janssen Research and Development.
https://www.nejm.org/doi/10.1056/NEJMoa2113194
Hina Zahid Joined Medical Dialogue in 2017 with a passion to work as a Reporter. She coordinates with various national and international journals and association and covers all the stories related to Medical guidelines, Medical Journals, rare medical surgeries as well as all the updates in the medical field. Email: editorial@medicaldialogues.in. Contact no. 011-43720751
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


