- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Single-Dose PanChol Shows Strong Safety and Immune Response in Phase 1 trial
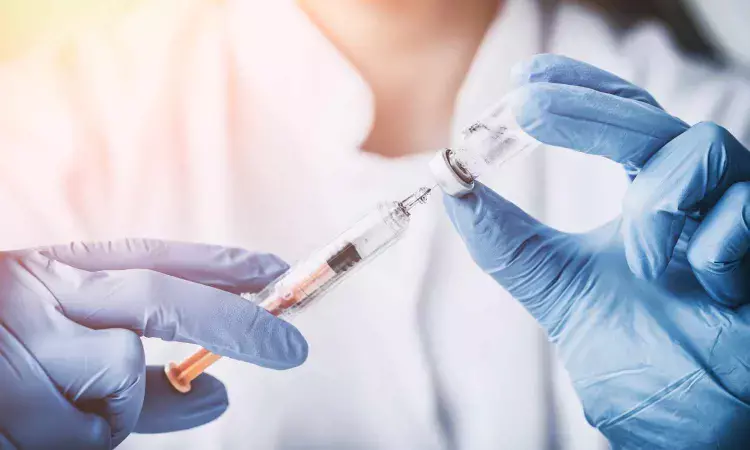
A new study published in the journal of The Lancet Infectious Diseases showed that over a 100,000-fold dosage range, a single oral dose of PanChol produced 100% vibriocidal seroconversion and was safe and well tolerated at all tested levels.
Killed whole-cell oral vaccinations are not very effective in young children and need to be administered in many doses. The current 7th pandemic strain of Vibrio cholerae O1 is the source of the single-dose, live-attenuated oral cholera vaccine known as PanChol. It overexpresses the non-toxic cholera toxin B subunit, co-expresses the Inaba and Ogawa antigens, and is engineered to reduce reactogenicity and prevent toxigenic reversion. Thus, this study evaluates PanChol's immunogenicity and safety in a first-in-human experiment.
The participants recruited were healthy people between the ages of 18 and 55 who had never had a cholera infection, had a vaccine, or had a gastrointestinal illness. Participants in the dose-escalation phase were given an extra dosage de-escalation module (10◦–10¹ CFU) in addition to a single oral dose of PanChol ranging from 10¹ to 10¹ CFU.
These participants were randomly assigned (7:7:4) to receive either a placebo or PanChol (2×10¹ or 2×10¹ CFU) during the blinded phase. Safety and vibriocidal seroconversion against Inaba and Ogawa strains at day 15 were co-primary objectives. Stool shedding was one of the secondary results.
57 healthy persons were enrolled between December 13, 2022, and February 7, 2025, where 15 in the dose-escalation module, 6 in the dose-de-escalation module, and 36 in the dose-expansion module (8 getting a placebo, 14 receiving 10¹ CFU PanChol). The majority of participants were White and non-Hispanic, with a median age of 30.6 years and 47% being male.
Everyone got the designated intervention. 38% of placebo receivers and 69% of PanChol recipients had solicited side events, most of which were moderate and temporary. The most frequent occurrence was diarrhea, which was reported at comparable rates in the placebo and vaccination groups. Only 4 incidents exceeded grade 2, and they were all unconnected to vaccination.
Uninvited adverse effects were common but often minor. Nearly all receivers of ≥10¹ CFU PanChol had stool shedding, whereas none received a placebo. Vibriocidal antibodies to both serotypes were seroconverted in all vaccination recipients with ≥10¹ CFU and accessible samples. Overall, PanChol was found to be safe and well-tolerated at all dosages, resulting in 100% vibriocidal seroconversion throughout a 100,000-fold dose range.
Source:
Leitner, D. R., Walsh, S. R., Suzuki, M., Desjardins, M., Hannaford, A., Sherman, A. C., Levine, H., Carr, L., Hammerness, E., Osaki, A., Sullivan, E., Wang, B., Balazs, G. I., Park Chang, J. B., Slater, D. M., Puri, N., Kuehl, C. J., Chen, W. H., Harris, J. B., … PanChol study group. (2026). Safety and immunogenicity of PanChol, a single-dose live-attenuated oral cholera vaccine: results from a phase 1a, double-blind, randomised, placebo-controlled trial. The Lancet Infectious Diseases. https://doi.org/10.1016/S1473-3099(25)00682-6
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


