- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Depot Medroxyprogesterone Acetate Use Linked to Higher Meningioma Risk in Women: JAMA
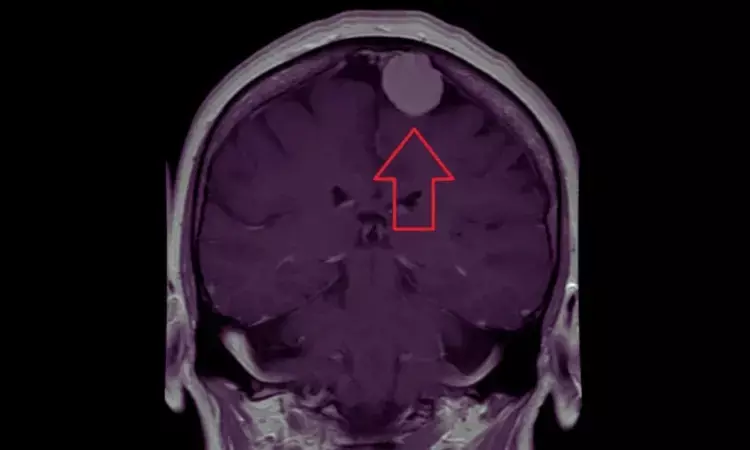
Researchers have found in a new study that women using depot medroxyprogesterone acetate had a higher risk of developing meningioma. Investigators have determined that women who used depot medroxyprogesterone acetate (DMPA) were at higher risk for the development of meningioma, especially with long-term use or when it was initiated at an older age. This finding is based on a large retrospective population-based cohort study that examined data in the TriNetX US national database from about 68 health care organizations from 2004 through 2024. The study was published in JAMA Neurology by Tianqi X. and colleagues.
The objective was to elucidate the risk of meningioma in women exposed to depot medroxyprogesterone acetate, oral medroxyprogesterone acetate, and other contraceptives like combined oral contraceptives, intrauterine devices (IUDs), progestin-only pills, and subdermal implants, versus women with no exposure to such agents.
The investigation had a cohort of 10,425,438 women with a mean age of 33.4 years at entry. The final analysis in propensity-score matched the final sample of 88,667 women on depot medroxyprogesterone acetate (mean age 26.2 years) against a matched control group. The main outcome was meningioma diagnosis identified through routine diagnostic codes. Relative risks (RRs) and number needed to harm (NNH) were estimated to evaluate clinical significance.
Results
• The results showed women who were administered depot medroxyprogesterone acetate to have a relative risk of 2.43 (95% CI, 1.77–3.33) for meningioma versus controls.
• This elevated risk was experienced mainly in those patients with greater than 4 years of treatment or initiators who began therapy at ages greater than 31 years.
• Oral medroxyprogesterone acetate had a less dramatic but statistically significant risk (RR 1.18; 95% CI, 1.10–1.27).
• No enhanced risk of meningioma was recognized in combined oral contraceptives, IUDs, progestin-only pills, or subdermal implants.
• The number needed to harm (NNH) was calculated to be 1,152 patients for depot medroxyprogesterone acetate and 3,020 patients for oral medroxyprogesterone acetate, showing that the absolute clinical risk is still quite low even with the relative increase in risk.
This big US-based study gives strong evidence that depot medroxyprogesterone acetate is related to an increased risk of meningioma, especially with long duration and advanced age at start. Nevertheless, the great number needed to harm implies that the absolute clinical risk is still low.
Reference:
Xiao T, Kumar P, Lobbous M, et al. Depot Medroxyprogesterone Acetate and Risk of Meningioma in the US. JAMA Neurol. Published online September 02, 2025. doi:10.1001/jamaneurol.2025.3011Dr Riya Dave has completed dentistry from Gujarat University in 2022. She is a dentist and accomplished medical and scientific writer known for her commitment to bridging the gap between clinical expertise and accessible healthcare information. She has been actively involved in writing blogs related to health and wellness.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


