- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
NPPA to Examine Separate Price Category for Cementless Knee Implants, Seeks Expert Inputs
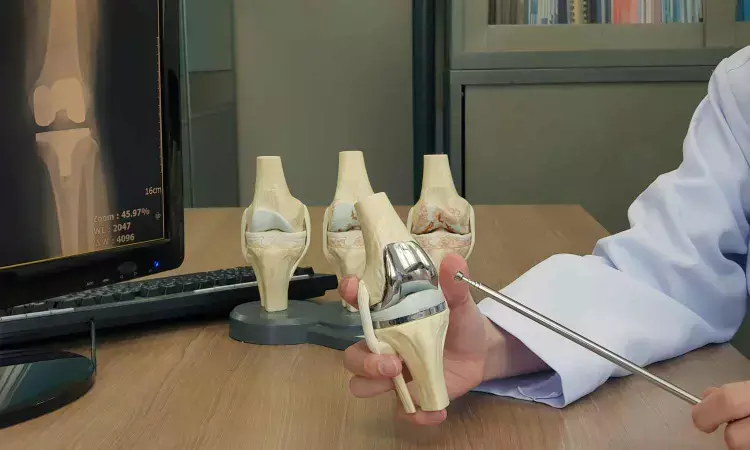
New Delhi: The National Pharmaceutical Pricing Authority (NPPA) is reviewing a request from knee implant manufacturers and importers to fix a separate ceiling price category for cementless knee implants.
The matter was taken up during the 68th meeting of the Multidisciplinary Committee of Experts held on June 3, 2025.
The Committee noted that NPPA had previously fixed ceiling prices for knee implants through a notification issued under Para 19 of the Drugs (Prices Control) Order (DPCO), 2013 vide S.O. 2668(E), dated 16th August 2017. Citing point 9(b) of the notification, the Committee referred to the following directive:
“All manufacturers shall comply and implement the ceiling prices mentioned hereinabove under the applicable category and serial number irrespective of being cementable, uncementable, gender specific or unisex. The manufacturer of uncemented implants, however, may approach NPPA with supporting documents, for fixation of separate ceiling price or retail price.”
Companies are now seeking a separate price category specifically for cementless knee implants. The Committee recalled that in its 48th meeting held on 14.08.2017, it was decided that:
“in case some innovative product by an overseas and/or Indian manufacturers is to be launched, which does not fall in any of the prescribed category or it has some ‘superiority’ over the existing implants, the companies will have option to explore the window provided under Para 11(3) & (4) of DPCO, 2013 for fixing a separate ceiling price or retail price as the case may be.”
Accordingly, the Committee has decided to co-opt subject experts from reputed medical institutions to examine the matter in detail. The experts will be drawn from “hospitals like All India Institute of Medical Sciences (AIIMS), Maulana Azad Medical College (MAMC), Sports Injury Centre (SIC) Safdarjung, Any Prominent Charitable Hospital and subject experts from NIPER for deliberation on this agenda.”
Farhat Nasim joined Medical Dialogue an Editor for the Business Section in 2017. She Covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She is a graduate of St.Xavier’s College Ranchi. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751


