- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Canadian health regulator actively reviewing Covaxin data, says Bharat Biotech US, Canada partner
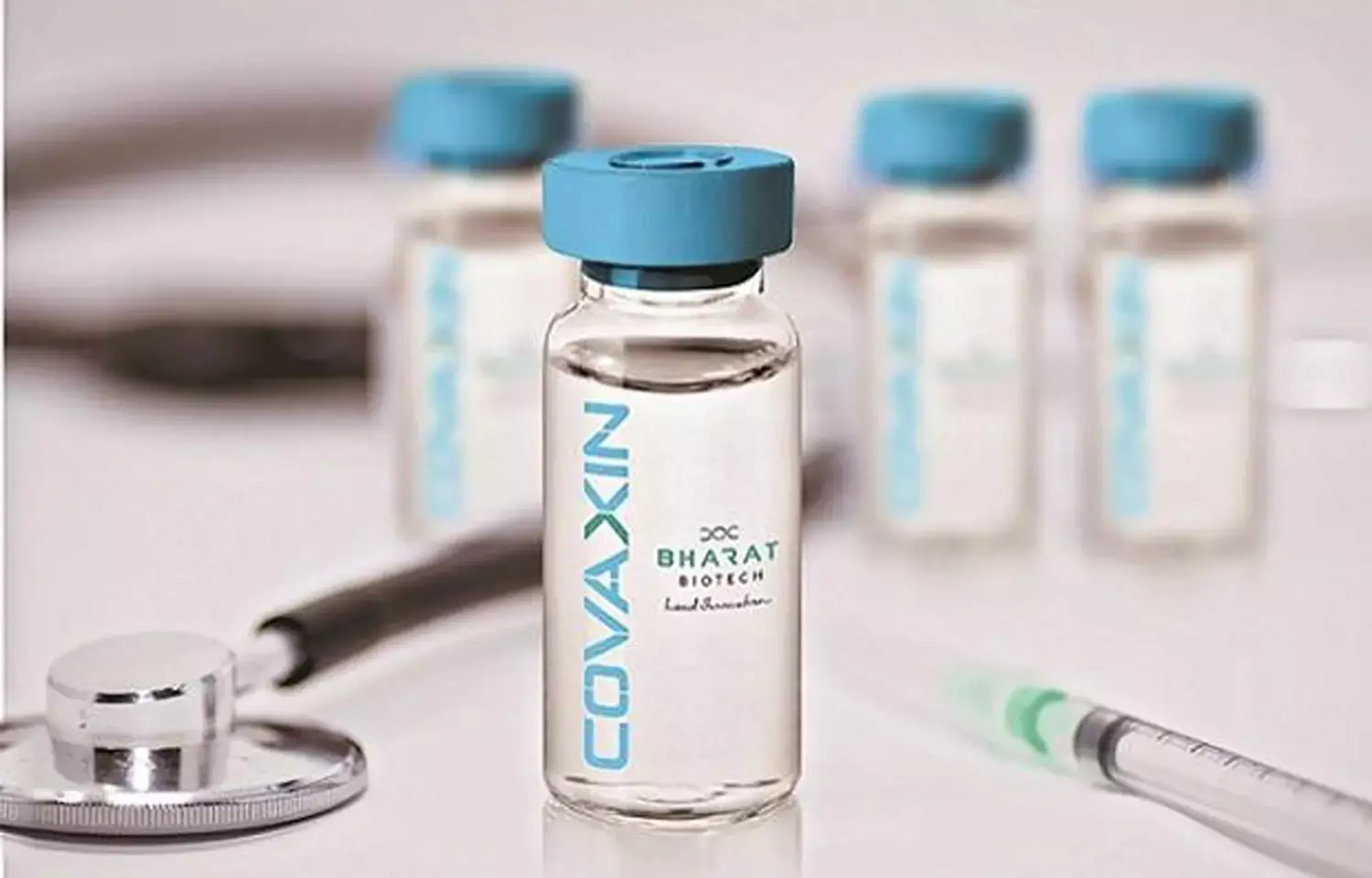
Hyderabad: Ocugen, the US and Canada partner of Bharat Biotech for Covaxin, has said it has submitted all data to the Canadian government and it is under active review of the health regulator there.
In June, Ocugen entered into an agreement with Bharat Biotech to develop, manufacture and commercialise the COVID-19 vaccine, Covaxin in Canada in addition to the existing rights in the United States. Ocugen has already approached Health Canada and submitted the Phase 3 clinical trial data of Covaxin seeking authorization to sell the jab in that country.
"We have submitted all the data, what is needed for the submission -- for Canadian submission. And again, on the specific timeline and the approval clock, we can't give you that at this stage," Shankar Musunuri--Chairman and Chief Executive Officer of Ocugen said on Friday in an Earnings call.
"All I can say is it's under active review by Health Canada. As we get questions, we're ready to respond to them very promptly and provide any information they need," he said.
Replying to a query he said Bharat Biotech is capable of supplying required quantities of Covaxin to USA and Canada as the Indian vaccine maker is ramping up production, targeting to produce more than half a billion doses per year.
Ocugen in a release said discussions with the US Food and Drug Administration are underway, and the company is still proceeding with a strategy focused on the agencys requested Biologics License Application pathway.
In a move that could potentially delay the launch of Covaxin in the US market, the FDA had earlier "recommended" Ocugen, to go for Biologics License Application (BLA) route with additional data, nixing hopes of Emergency Use Authorisation for the jab.
"We have most of the data from Phase III clinical trial, including all the manufacturing. We are still discussing the regulatory path for the BLA, what is required, if any, additional studies," Musunuri said.
Read also: Bharat Biotech partner initiates rolling submission to Health Canada for Covaxin
Ruchika Sharma joined Medical Dialogue as an Correspondent for the Business Section in 2019. She covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She has completed her B.Com from Delhi University and then pursued postgraduation in M.Com. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751


