- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Pfizer teams up with Gilead to help manufacture Covid drug remdesivir
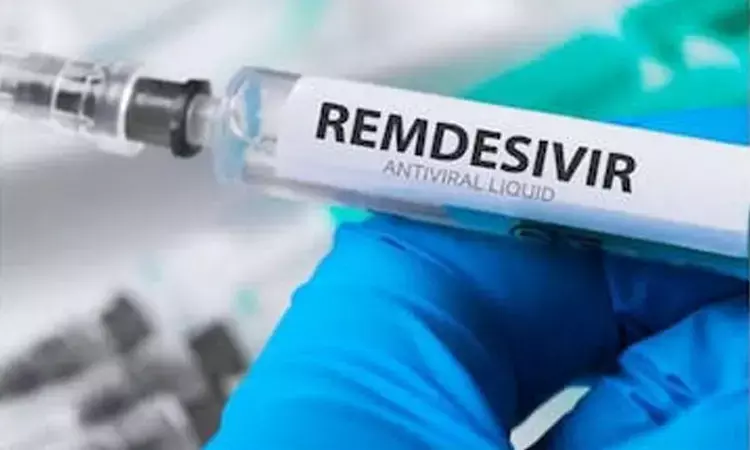
US: Pfizer Inc (PFE.N) said on Friday it signed a multiyear agreement to make COVID-19 treatment remdesivir for developer Gilead Sciences Inc (GILD.O), which is under pressure to increase tight supplies of the antiviral drug.
Gilead is aiming to make enough of the drug by the end of the year to treat more than 2 million COVID-19 patients and agreed to send nearly all of its remdesivir supply to the United States through September.
But hospital staffers and politicians have complained about difficulties in gaining access to the drug, which is one of only two to have demonstrated an ability to help hospitalized COVID-19 patients in formal clinical trials.
There are also fears of shortages outside the United States, and separately on Friday, Britain's Hikma Pharmaceuticals Plc (HIK.L) said it has started manufacturing remdesivir at its Portugal plant.
Gilead said its manufacturing network for the drug had grown to more than 40 companies in North America, Europe and Asia to add capacity.
Earlier this week, a bipartisan group of U.S. state attorneys general urged the federal government to allow other companies to make Gilead's remdesivir, to increase its availability and lower the price of the antiviral drug.
Pfizer will provide contract manufacturing services through its McPherson, Kansas, plant, the drugmaker said. It was not immediately clear if Pfizer would supply only for the U.S. market.
The U.S. Food and Drug Administration sent a warning letter to Pfizer in 2017 saying that the process for manufacturing sterile injectable drugs at the Kansas plant was "out of control" and put patients at risk.
The FDA said several products were contaminated with multiple foreign particulates but a subsequent FDA inspection found that the issues had been resolved.
Pfizer, with Germany's BioNTech (22UAy.F), is also rushing to develop a vaccine against the coronavirus.
Pfizer has helped other drugmakers manufacture their products before. It makes Epipen emergency allergy treatments through its Meridian Medical Technologies business and also operates a contract manufacturer called Center One.
Read also: Jubilant Life Sciences Launches Remdesivir Under Brand Name JUBI-R In Indiahttps://medicaldialogues.in/news/industry/pharma/jubilant-life-sciences-launches-remdesivir-under-brand-name-jubi-r-in-india-68253
Ruchika Sharma joined Medical Dialogue as an Correspondent for the Business Section in 2019. She covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She has completed her B.Com from Delhi University and then pursued postgraduation in M.Com. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751


