- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Study observes favourable safety profile of dupilumab in pregnancy
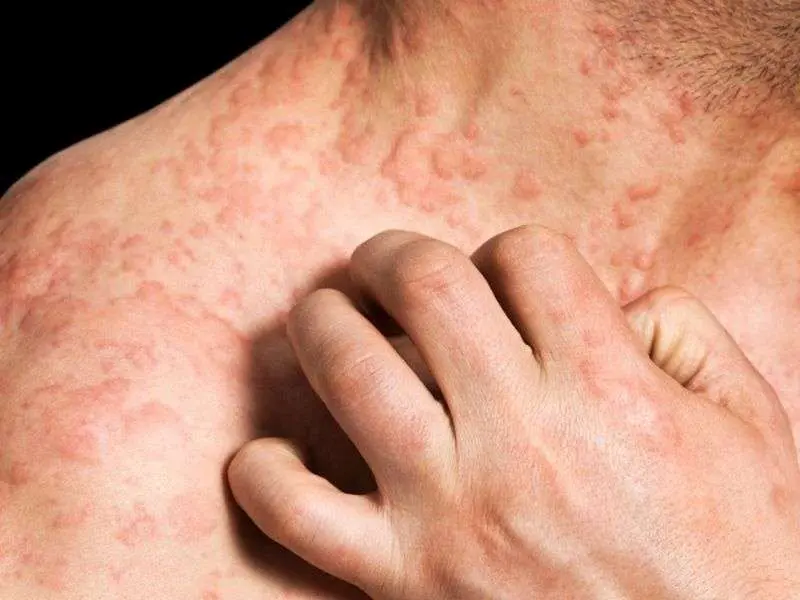
A new study published in the Journal of the European Academy of Dermatology and Venerology found dupilumab to have a favorable safety profile during pregnancy.
Chronic type 2 inflammatory disorders (T2IDs) afflict around 20% of the general population, with many of these conditions affecting women who are of reproductive age. The immune system changes to Th2 dominance during pregnancy, which frequently exacerbates or causes T2IDs such as bronchial asthma and pregnancy-related atopic eruption.
Due to the dearth of authorized pregnancy drugs, systemic therapy choices are still restricted, despite the fact that effective treatment is essential to lowering risks for both mother and child. Although biologics such as dupilumab have a strong track record in the general population, there is not enough information on their safety during pregnancy to provide firm recommendations or get official clearance at this time.
TriNetX's US Collaborative Network provided pregnant women with T2ID who were receiving dupilumab during their pregnancy. The controls were pregnant T2ID patients who were not receiving dupilumab medication. For demographics, diagnoses, medicines, and potential risk factors for APO, propensity score matching (PSM) was used.
Premature obstetric labor, pregnancy-induced hypertension, gestational diabetes, puerperal infections, and spontaneous abortion were among the outcomes that were examined. The Kaplan-Meier technique, the log-rank test for outcome differences, and the Cox regression model's hazard ratios (HR) were used to evaluate survival studies.
Dupilumab was administered to a total of 293 pregnant women. No elevated risks for APOs were seen after PSM. Notably, the group treated with dupilumab had lower chances for "any APO" and early obstetric labor. Additionally, up to 6 months prior to pregnancy and throughout the postpartum phase, there was no discernible difference in the risks for any APO between women receiving dupilumab and those not receiving it.
Overall, in the absence of prospective data, the results of this investigation merit clinical evaluation. This is the first large-scale cohort research to compare pregnant women receiving dupilumab medication to those not receiving it in terms of their risk of APOs. This study confirmed dupilumab's safety for maternal APOs by finding no elevated risk and even a decreased risk for several APOs in the group treated with it.
Reference:
Preuß, S. L., Bieber, K., Vorobyev, A., Recke, A., Moderegger, E. L., Zirpel, H., Gaffal, E., Thaçi, D., Kridin, K., & Ludwig, R. J. (2025). Dupilumab shows no elevated risk for maternal adverse pregnancy outcomes: A propensity-matched cohort study. Journal of the European Academy of Dermatology and Venereology: JEADV. https://doi.org/10.1111/jdv.20670
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


