- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Investigational magnetic device shrinks glioblastoma in first-in-world human test
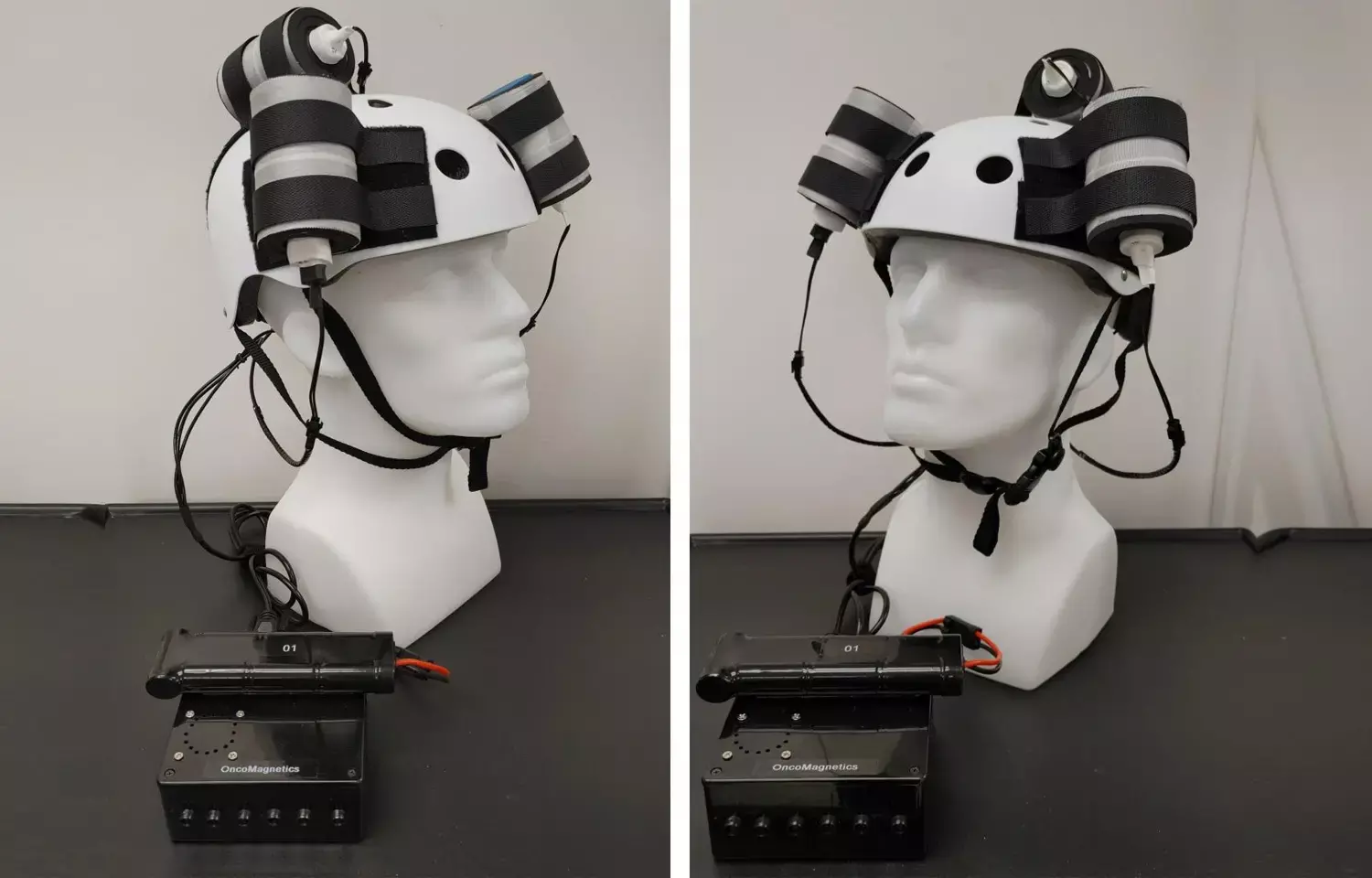
Houston Methodist Neurological Institute researchers from the department of neurosurgery shrunk a deadly glioblastoma tumor by more than a third using a helmet generating a noninvasive oscillating magnetic field that the patient wore on his head while administering the therapy in his own home. The 53-year-old patient died from an unrelated injury about a month into the treatment, but during that short time, 31% of the tumor mass disappeared. The autopsy of his brain confirmed the rapid response to the treatment.
"Thanks to the courage of this patient and his family, we were able to test and verify the potential effectiveness of the first noninvasive therapy for glioblastoma in the world," said David S. Baskin, M.D., FACS, FAANS, corresponding author and director of the Kenneth R. Peak Center for Brain and Pituitary Tumor Treatment in the Department of Neurosurgery at Houston Methodist. "The family's generous agreement to allow an autopsy after their loved ones' untimely death made an invaluable contribution to the further study and development of this potentially powerful therapy."
In a case study published in Frontiers in Oncology Baskin and his colleagues detailed the journey of their pioneering patient who suffered from end-stage recurrent glioblastoma, despite a radical surgical excision, chemoradiotherapy and experimental gene therapy.
Glioblastoma is the deadliest of brain cancers in adults, nearly always fatal, with a life expectancy of a few months to two years. When the patient's glioblastoma recurred in August 2019, Baskin and his team, already working on the OMF treatment in mouse models, received FDA approval for compassionate use treatment of the patient with their newly invented Oncomagnetic Device under an Expanded Access Program (EAP). The protocol also was approved by the Houston Methodist Research Institute Institutional Review Board.
The treatment consisted of intermittent application of an oscillating magnetic field generated by rotating permanent magnets in a specific frequency profile and timing pattern. First administered for two hours under supervision in the Peak Clinic, ensuing treatments were given at home with help from the patient's wife, with increasing treatment times up to a maximum of only six hours per day.
The Oncomagnetic Device looks deceptively simple: three oncoscillators securely attached to a helmet and connected to a microprocessor-based electronic controller operated by a rechargeable battery, an invention by case study co-author Dr. Santosh Helekar. During the patient's five weeks of treatment, the magnetic therapy was well-tolerated and the tumor mass and volume shrunk by nearly a third, with shrinkage appearing to correlate with the treatment dose.
Co-authored by associate professor of neurosurgery Santosh Helekar, M.D., Ph.D., research professor Martyn A. Sharpe, Ph.D., and biomedical engineer Lisa Nguyen, the case study is entitled "Case Report: End-Stage Recurrent Glioblastoma Treated with a New Noninvasive Non-Contact Oncomagnetic Device." The ongoing research is supported by the Translational Research Initiative of the Houston Methodist Research Institute, Donna and Kenneth Peak, the Kenneth R. Peak Foundation, the John S. Dunn Foundation, the Taub Foundation, the Blanche Green Fund of the Pauline Sterne Wolff Memorial Foundation, the Kelly Kicking Center Foundation, the Gary and Marlee Swarz Foundation, the Methodist Hospital Foundation and the Veralan Foundation.
"Imagine treating brain cancer without radiation therapy or chemotherapy," said Baskin. "Our results in the laboratory and with this patient open a new world of non-invasive and nontoxic therapy for brain cancer, with many exciting possibilities for the future."
For further references log on to:
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


