- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Faricimab reduces treatment burden of neovascular age-related macular degeneration: Lancet
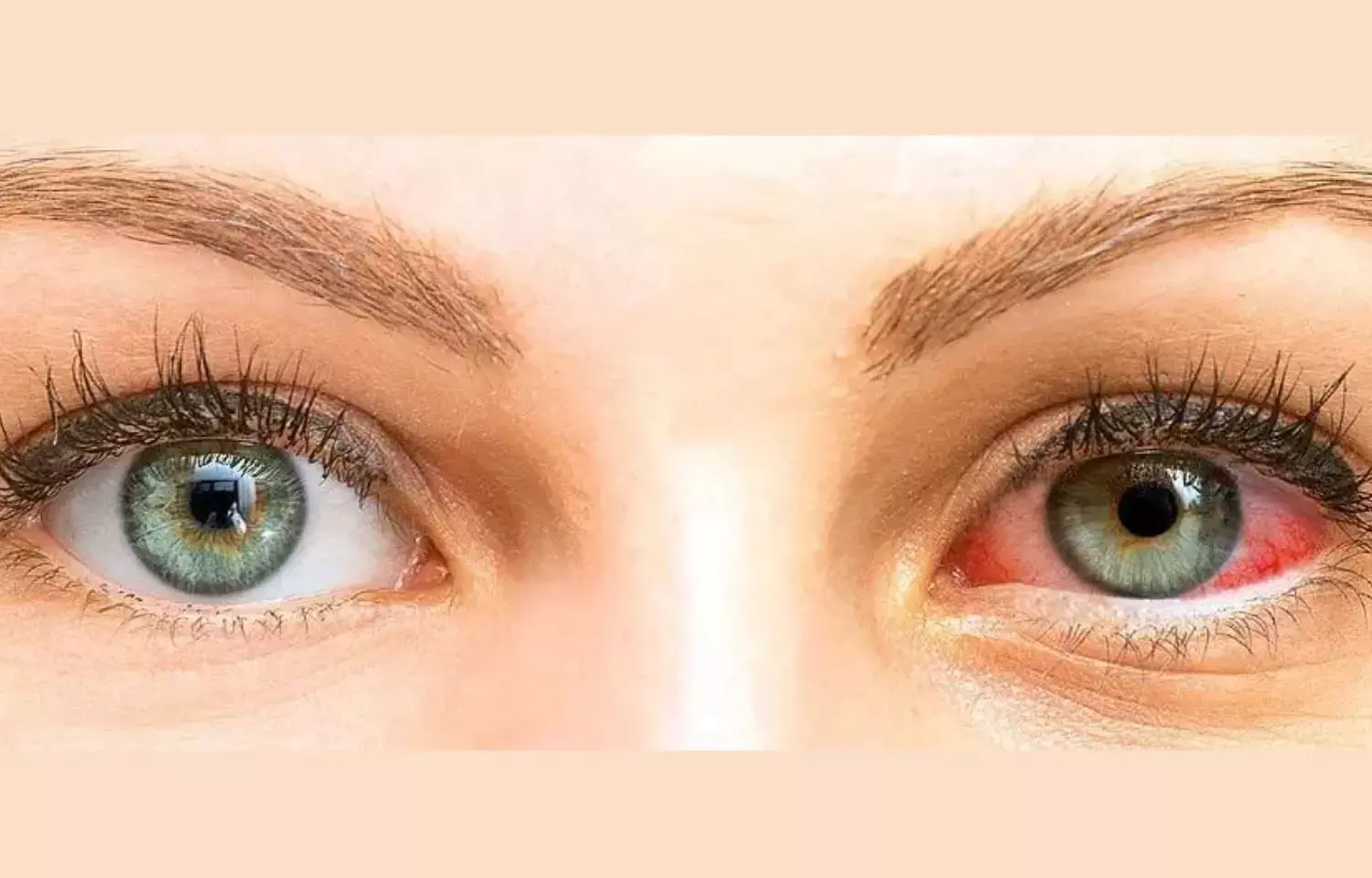
A new study published in The Lancet suggests that Faricimab can effectively prolong the duration between treatments with continued efficacy and reduce treatment burden in patients with neovascular age-related macular degeneration (nAMD).
A bispecific antibody called faricimab works by simultaneously inhibiting angiopoietin-2 and vascular endothelial growth factor A. Jeffrey S. Heier and colleagues provide the initial findings of two phase 3 studies testing intravitreal faricimab for neovascular age-related macular degeneration, with extensions up to every 16 weeks.
Randomized, double-masked studies for TENAYA and LUCERNE were conducted at 271 locations around the globe. Based on protocol-defined disease activity evaluations at weeks 20 and 24, treatment-naive individuals with nAMD 50 years of age or older were randomly allocated to intravitreal faricimab 6 mg up to every 16 weeks or aflibercept 2 mg every 8 weeks. Patients, researchers, those who were evaluating the results, and the funders were all blinded to the group allocations. The primary endpoint of this study was the mean change in best-corrected visual acuity (BCVA) from baseline averaged across weeks 40, 44, and 48. Patients who had at least one dosage of trial therapy included in the safety analysis.
The key findings of this study were as follow:
1. Between February 19 and November 19, 2019 (TENAYA n=334 faricimab and n=337 aflibercept) and to March 11 and November 1, 2019 (LUCERNE n=331 faricimab and n=327 aflibercept), 1329 patients were randomly allocated between the two studies.
2. In both TENAYA and LUCERNE, the BCVA change from baseline with faricimab was non-inferior to aflibercept.
3. Faricimab and aflibercept both had similar rates of ocular side effects.
In conclusion, the key findings of this study suggest that patients with neovascular age-related macular degeneration who are both difficult to treat and treatment-naive are responding favorably to faricimab.
Reference:
Heier, J. S., Khanani, A. M., Quezada Ruiz, C., Basu, K., Ferrone, P. J., Brittain, C., Figueroa, M. S., Lin, H., Holz, F. G., Silverman, D., Abdulaeva, E., … Zeolite, C. (2022). Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. In The Lancet (Vol. 399, Issue 10326, pp. 729–740). https://doi.org/10.1016/s0140-6736(22)00010-1
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


