- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA Approves Prednisolone Acetate Ophthalmic Suspension for Ocular Inflammation
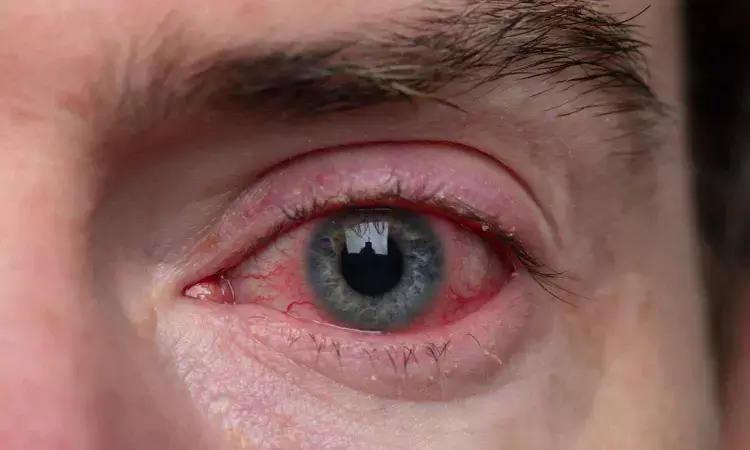
The FDA has approved preodnisolone acetate ophthalmic suspension, USP 1%, for treating steroid-responsive ocular inflammation. Amneal Pharmaceuticals plans to launch the product in Q3 2025.
Prednisolone acetate ophthalmic suspension, USP 1% is a sterile, topical anti-inflammatory 0agent for ophthalmic use and is indicated for treating steroid-responsive ocular inflammation.
“Our Affordable Medicines portfolio continues to grow with a strong and diverse pipeline that supports broader access to high-quality treatments across the U.S. healthcare system,” said Andy Boyer, Executive Vice President and Chief Commercial Officer, Affordable Medicines. “The approval of prednisolone acetate ophthalmic suspension-a complex product to develop and manufacture-highlights the depth of our R&D capabilities and the strength of our manufacturing and supply operations.”
The most commonly reported adverse reactions for prednisolone acetate ophthalmic suspension in clinical studies were elevation of intraocular pressure (IOP) with possible development of glaucoma and infrequent optic nerve damage, posterior subcapsular cataract formation, and delayed wound healing. For prescribing information, see package insert here.
According to IQVIA® U.S. annual sales for prednisolone acetate ophthalmic suspension for the 12 months ended April 2025 were approximately $201 million.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


