- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA approves ranibizumab for treating diabetic macular edema
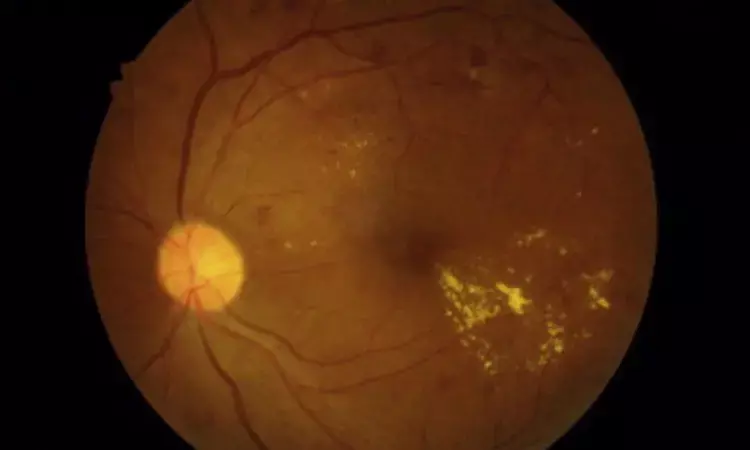
The U.S. Food and Drug Administration (FDA) has approved ranibizumab injection (Susvimo, 100 mg/mL; Genentech) for the treatment of diabetic macular edema (DMO). According to the company, Susvimo is the first and only FDA-approved treatment proven to maintain vision in people with DMO while requiring fewer treatments compared to standard eye injections.
“Susvimo presents a unique, convenient treatment alternative to routine eye injections for people with a potentially blinding diabetic eye condition,” said Levi Garraway, M.D., Ph.D., chief medical officer and head of Global Product Development. “As the global prevalence of this condition continues to grow, today's FDA approval for Susvimo reflects our dedication to innovation and enhancing the patient experience.”
“I am excited to offer Susvimo to my patients living with diabetic macular edema who want an option with longer intervals between treatments due to their busy personal and professional lives,” said vitreoretinal surgeon, Jordan Graff, M.D., Barnet Dulaney Perkins Eye Center, Arizona. “Having completed dozens of Susvimo surgeries in my patients with wet, or neovascular, age-related macular degeneration, I’ve seen firsthand how Susvimo, with its continuous delivery of medication, can help preserve vision with fewer treatments. I look forward to broadening Susvimo’s impact to even more patients in my clinic.”
The FDA decision was based on positive one-year results from the Phase III Pagoda study, which showed that Susvimo demonstrated sustained vision improvements in people with DME, with safety consistent with the known safety profile for Susvimo. In Pagoda, people with DME who received Susvimo refilled every six months achieved non-inferior improvements in vision compared with those receiving monthly 0.5 mg ranibizumab intravitreal injections (9.6 eye chart letters, similar to gaining two more lines on an eye chart, compared to 9.4 letters, respectively).
Susvimo provides continuous delivery of a customized formulation of ranibizumab via the Port Delivery Platform, while other currently approved treatments may require eye injections as often as once per month. Susvimo was first approved by the FDA for the treatment of wet, or neovascular age-related macular degeneration (AMD) in 2021.
Genentech is committed to helping people access the medicines they are prescribed and will be offering comprehensive services for people prescribed Susvimo to help minimize barriers to access and reimbursement. Patients can call 833-EYE-GENE for more information. For people who qualify, Genentech offers patient assistance programs through Genentech Access Solutions.
About Diabetic Macular Edema (DME)
Affecting approximately 750,000 people in the U.S. and 29 million people globally, diabetic macular edema (DME) is a vision-threatening retinal condition associated with blindness and decreased quality of life when left untreated. DME occurs when damaged blood vessels in the retina leak into and cause swelling in the macula – the central area of the retina responsible for the sharp vision needed for reading and driving. The number of people with DME is expected to grow as the prevalence of diabetes increases.
About Susvimo® (ranibizumab injection) 100 mg/mL for intravitreal use via ocular implant
Susvimo® (ranibizumab injection) 100 mg/mL for intravitreal use via ocular implant is a refillable implant surgically inserted into the eye during a one-time, outpatient procedure. Susvimo continuously delivers a customized formulation of ranibizumab over time. Susvimo is indicated for intravitreal use via the Susvimo eye implant only. Ranibizumab is a vascular endothelial growth factor (VEGF) inhibitor designed to bind to and inhibit VEGF-A, a protein that has been shown to play a critical role in the formation of new blood vessels and the leakiness of the vessels. Susvimo was previously called the Port Delivery System with ranibizumab in the U.S.
The customized formulation of ranibizumab delivered by Susvimo is different from the ranibizumab intravitreal injection, a medicine marketed as Lucentis® (ranibizumab injection), which is approved to treat wet, or neovascular, age-related macular degeneration (AMD) and other retinal diseases. Lucentis was first approved for wet AMD by the FDA in 2006. Genentech is also developing DutaFabs – the next generation of bispecific antibodies designed for increased efficacy and durability – tailored for continuous delivery via the Port Delivery implant.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


