- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Loteprednol etabonate formulated with MPP technology effective in dry eye disease
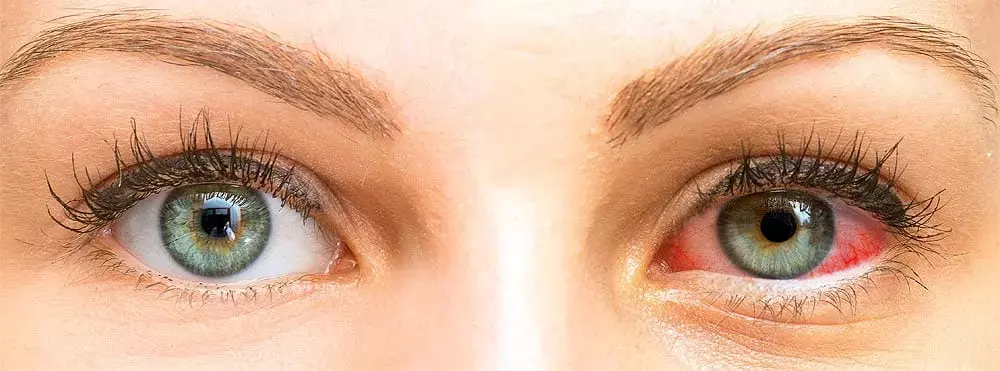
USA: A review, published in the Dove press journal Clinical Ophthalmology, has described the use of a commercially available formulation of loteprednol etabonate ophthalmic suspension (0.25%) for the treatment of acute symptoms of dry eye disease (DED). It found that the unique formulation is well-suited for dry eye flare-ups.
Dry eye disease is a prevelant disease of ocular surface. Patients with DED can get episodic flares, like any chronic disease. For the chronic treatment of DED, there are many existing and upcoming treatments, yet treatments for DED flares are limited. Loteprednol etabonate 0.25% is approved by FDA for the short-term treatment of the signs and symptoms of DED.
Formulation of this medications is with the customized mucus-penetrating particle (MPP) technology that has a significant ability to penetrate the ocular surface for effective deliver of the active steroid to the ocular surface tissues as compared with conventional steroid preparations. The MPP technology involves engineering nanoparticles designed to effectively penetrate mucus and prevent entrapment of drug particles by mucins. Also, there is an increasing use of loteprednol etabonate 0.25% for the treatment of DED before and/or after cataract or refractive surgery or as induction therapy before starting chronic immunomodulatory medication for DED.
There has been a focus on understanding the disease pathophysiology and identifying effective treatment modalities given the significant prevalence and morbidity of DED.
Nandini Venkateswaran, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, MA, USA, and colleagues conclude, "KP-121 0.25% with its custom engineered MPP technology allows for the effective and efficient delivery of loteprednol etabonate onto the ocular surface for the short-term treatment of signs and symptoms of DED with minimal side effects."
"Clinical trials have demonstrated the utility of this medication in DED flares and clinicians should incorporate this medication into their DED treatment armamentarium," they wrote.
Reference:
Venkateswaran N, Bian Y, Gupta PK. Practical Guidance for the Use of Loteprednol Etabonate Ophthalmic Suspension 0.25% in the Management of Dry Eye Disease. Clin Ophthalmol. 2022;16:349-355
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


