- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Making Better-Informed Decisions in Glaucoma: JAMA Ophthalmology
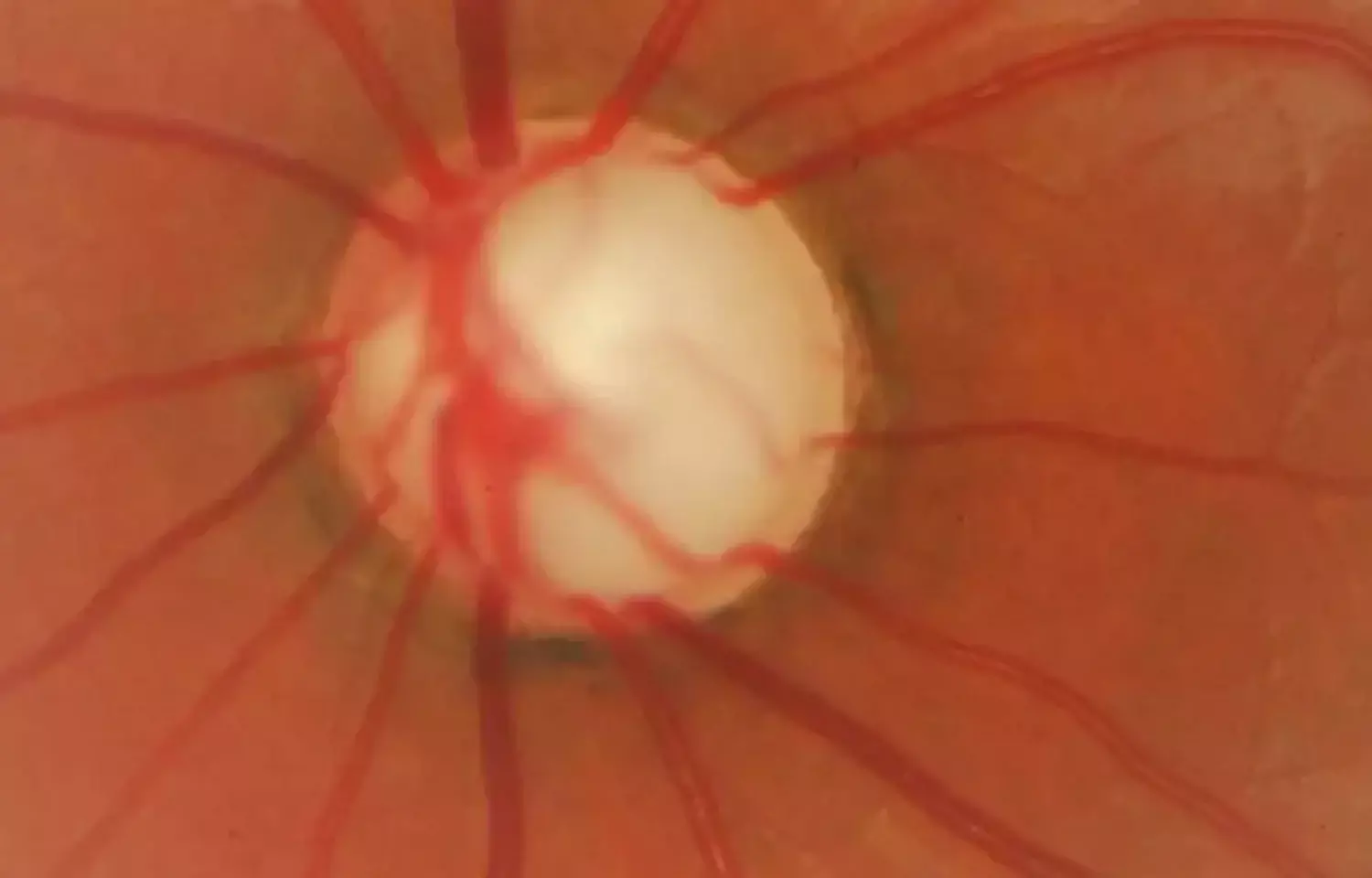
Making decisions in glaucoma management often is difficult.
1. How can we best detect progression expeditiously?
2. How well does structural progression correlate with reduction in eye-related quality of life? After all, preserving function and quality of life is the goal.
These questions are addressed by Nishida et al in a collaboration of leading investigators of the use of imaging in the detection of glaucoma and glaucoma progression. Overall, 236 eyes of 118 individuals were enrolled in 2 prospective studies and underwent regularly scheduled measurements of ganglion cell complex thickness, visual fields, and vision-related quality of life surveys.
This article by Paul F. Palmberg addresses the problem of how to expeditiously detect meaningful glaucomatous progression, where detecting the signal of change is difficult given the noise in detection methods.
In visual field testing, the problem is variability due to random fluctuation in patient responses and/or true day-to-day changes in the ability of stressed ganglion cells to respond. In structural measurement, the problem is that there is a relatively wide range of tissue thickness of the retinal nerve fiber layer or macular ganglion cell complex prior to injury so that a person can experience loss without falling below a statistical normal limit unless the damage is sufficiently focal. Thus, while the thickness of structure can be reliably measured, change in structure is more readily detected as evidence of damage.
Their result? The work shows that faster change in the macular ganglion cell complex correlates with vision-related quality-of-life measures, albeit weakly (R2 = 0.2, ie, only 20% of the change in quality of life is attributable to the change in ganglion cell complex thickness) throughout the short term of a few years.
A post hoc analysis was performed comparing the 10 visual fields obtained during the first 5 years of treatment with 2 baseline pretreatment visual fields. That analysis revealed 2 important messages.
First, in the group of participants with all IOP levels 17 mm Hg or lower, only 6% of fields were worse by 3 dB of mean deviation, the same as the measured 6% variability of baseline testing, well below the 20% of participants in the entire study. Second, the lower the peak pressure, the more likely a measured 3 dB of mean deviation improvement occurred, particularly for a peak IOP of 13 mm Hg. In other words, with lower IOP, the risk of progression fell to the level of the noise of testing, and the lower the IOP, the greater the chance that dysfunctional ganglion cells recovered function. Still, it was in a minority of tests that a 3-dB improvement was observed, with a net of 12% better than worse.
While the clinical trials have shown that aggressive treatment is highly effective in preventing progression, the CIGTS results also suggest that only 1 in 8 similar individuals actually require a low normal IOP to obtain the optimal result of stability plus a chance for significant recovery of visual field. Getting there to benefit some could mean creating surgical complications for others. If one instead chooses to observe in a higher pressure range while receiving safer therapies, some patients will experience significant progression before can detect it and act. Therein lies the dilemma.
Maria Francesca Cordeiro, MD, PhD, of Imperial College London, has published clinical trials in which stressed or dying ganglion cells are imaged and counted using annexin V coupled with a fluorescent probe. The basis for the test is that the binding sites for the structural protein annexin V come to the surface of ganglion cells as they begin to commit to an apoptotic death, the pathway by which ganglion cells die in glaucoma. Administration of the annexin V fluorescent probe intravenously results in maximum staining of such cells at 2 hours after administration. In an initial human trial, the test agent was shown to be safe, and the trial resulted in more stained cells in patients with glaucoma than in normal controls, with the number of cells stained correlating with the IOP and with recent progression.
The technique was then augmented by a convoluted neural network–derived algorithm far more sensitive in detecting the cells at a level of stain above background signal, and the algorithm also automatically performed the cell count from standard optical coherence tomography angiography images. The number of stained cells was shown to statistically significantly predict which patients, under usual glaucoma treatment and IOPs in the upper normal range, went on to significant loss of retinal nerve fiber layer. This ganglion cell "screamometer" needs to be expeditiously validated in regulatory studies. If its promise is confirmed, one can look forward to practicing precision medicine, fine tuning treatments to optimize IOP-related ganglion cell function, and better informing the risk/benefit assessment.
Source: Paul F. Palmberg; Association Between Ganglion Cell Complex Thinning and Vision-Related Quality of Life in Glaucoma; Volume 140, Number 8 doi:10.1001/jamaophthalmol.2022.2141
Dr Ishan Kataria has done his MBBS from Medical College Bijapur and MS in Ophthalmology from Dr Vasant Rao Pawar Medical College, Nasik. Post completing MD, he pursuid Anterior Segment Fellowship from Sankara Eye Hospital and worked as a competent phaco and anterior segment consultant surgeon in a trust hospital in Bathinda for 2 years.He is currently pursuing Fellowship in Vitreo-Retina at Dr Sohan Singh Eye hospital Amritsar and is actively involved in various research activities under the guidance of the faculty.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


