- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Intra articular Diclofenac-hyaluronate conjugate effective in relieving hip osteoarthritis pain
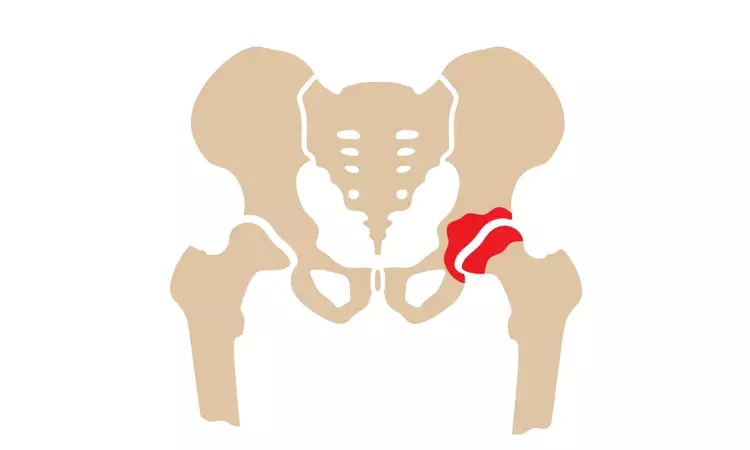
When delivered every four weeks, intra-articular diclofenac etalhyaluronate (DF-HA) provided a quick response and was safe, with analgesia lasting 12 weeks in patients with osteoarthritis (OA) of the hip, says an article published in BMC Musculoskeletal Disorders.
Osteoarthritis is a joint condition that commonly affects middle-aged and older persons, lowering their quality of life (QOL) by interfering with everyday activities due to pain, swelling, and deformity. OA may affect every joint in the body, including the hip, knee, shoulder, ankle, and elbow. Diclofenac etalhyaluronate (DF-HA), which will be licensed in Japan in March 2021 for the treatment of knee and hip OA, is a new intra-articular injectable drug composed of fermentation-derived HA (600,000 to 1,200,000 Da) chemically coupled with diclofenac sodium (DF), an NSAID.
Toshikazu Kubo and colleagues designed this study to assess the efficacy and safety of intra-articular DF-HA provided every 4 weeks in joints with OA other than the knee, and in particular to see if DF-HA had comparable efficacy and safety profiles in other joints afflicted by OA as the knee.
Japanese patients aged 20 years reported with OA of the ankle, hip, shoulder, or elbow were randomly allocated 1:1 to DF-HA 30 mg or placebo in this randomized, placebo-controlled, double-blind trial in Japan (citric acid-sodium citrate buffered solution). Subjects were examined 12 weeks after the first injection and received three injections of the study medication in each joint cavity every four weeks. The primary objective was the mean change from baseline in a 12-week diary-based 11-point numerical rating scale (NRS) for pain. Treatment-emergent side effects were documented, and radiographic changes in each joint were assessed.
The key findings of this study were as follow:
1. The research treatment (DF-HA versus placebo) was given to 90, 60, 90, or 50 people with hip, ankle, shoulder, or elbow OA (46 vs 44, 30 vs 30, 45 vs 45, and 25 vs 25, respectively).
2. The mean change from baseline in the pain NRS over 12 weeks for the hip, ankle, shoulder, and elbow joints was -0.81, -0.07 (-1.03 to 0.89), 0.15 (-0.48 to 0.78), and 0.61 (-0.41 to 1.62), with statistically significant changes detected only in the hip joint.
3. At all time intervals from Weeks 1 to 12, the change from baseline in the hip joint was larger with DF-HA than with placebo and there were no clinically significant adverse effects or radiological abnormalities.
Reference:
Kubo, T., Kumai, T., Ikegami, H., Kano, K., Nishii, M., & Seo, T. (2022). Diclofenac–hyaluronate conjugate (diclofenac etalhyaluronate) intra-articular injection for hip, ankle, shoulder, and elbow osteoarthritis: a randomized controlled trial. In BMC Musculoskeletal Disorders (Vol. 23, Issue 1). Springer Science and Business Media LLC. https://doi.org/10.1186/s12891-022-05328-3
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


