- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Switching to IV golimumab from IV infliximab improves disease activity in RA
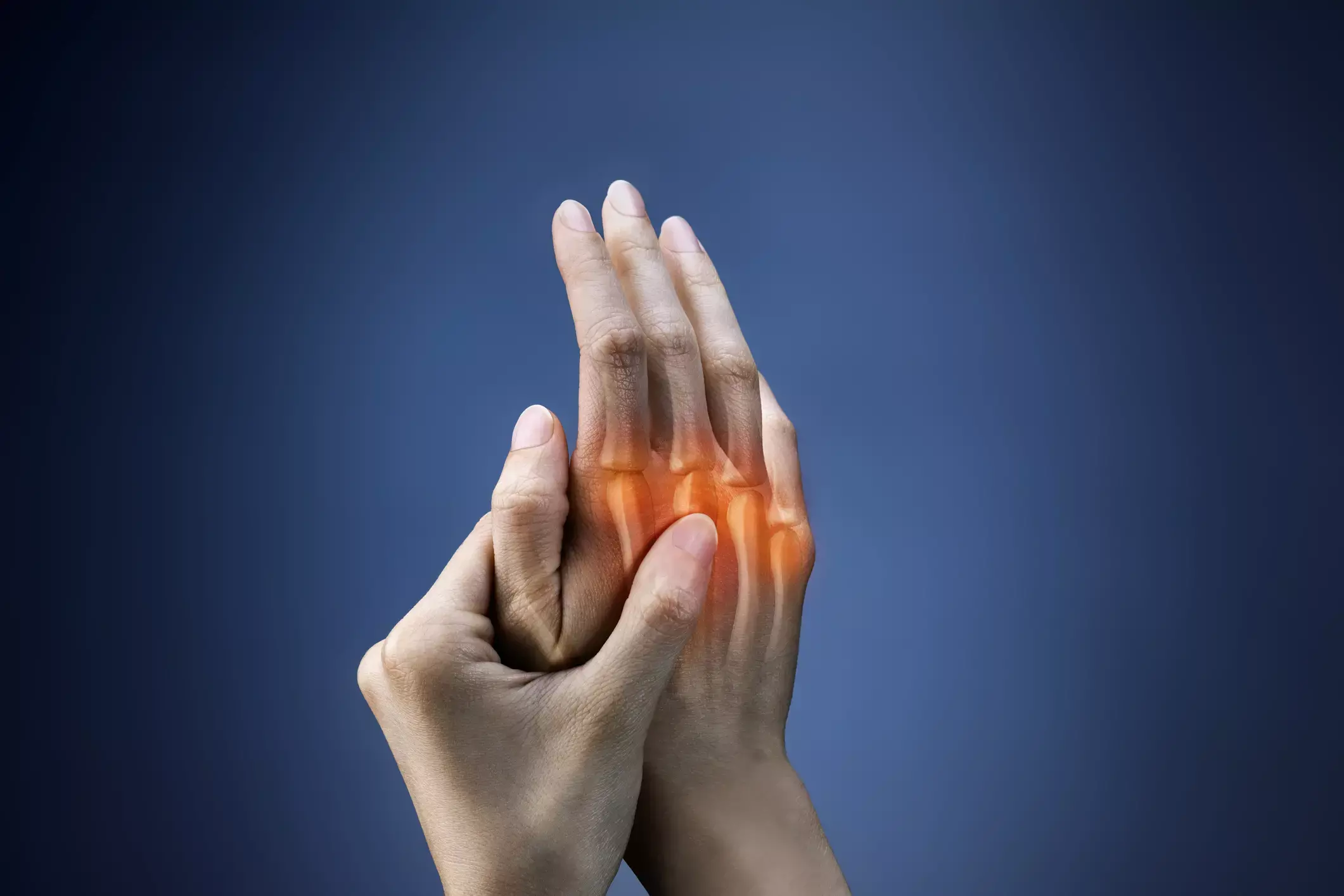
USA: Switching to IV-golimumab from IV-infliximab is beneficial in patients with rheumatoid arthritis (RA) as it may improve drug tolerance and disease activity, reveals a recent study involving real-world patients.
"Results of the study using RISE data indicate the effectiveness of IV-golimumab in improving RA disease activity in a patient population switching directly from IV-infliximab," John Tesser, Arizona Arthritis & Rheumatology Associates, Phoenix, AZ, USA, and colleagues wrote in their study published in the journal Clinical Rheumatology. This was measured by a standard disease activity index aligned with ACR and EULAR treat-to-target guidelines called CDAI.
"These results are clinically meaningful and may be helpful in providing patients with improved and sustained outcomes given the limited real-world data reporting the efficacy of IV-infliximab-to-IV-golimumab switching."
Golimumab and infliximab are intravenously (IV) administered tumor necrosis factor inhibitors approved for the treatment of moderate-to-severe rheumatoid arthritis with concomitant methotrexate. However, due to the differences in their biological construct, patients with rheumatoid arthritis (RA) with a failure response to IV-infliximab may benefit from switching to IV-golimumab.
Using real-world data from ACR's Rheumatology Informatics System for Effectiveness (RISE), Dr. Tesser and colleagues aimed to evaluate the efficacy of switching from IV-infliximab to IV-golimumab to control RA disease activity. RISE is a large electronic health records registry based in the USA.
The longitudinal, retrospective, single-arm study included adults with ≥ 1 RA diagnosis code between 2014 and 2018 and ≥ 1 IV-infliximab prescription within 6 months of a new IV-golimumab order (index date). Clinical Disease Activity Index (CDAI) was used to calculate longitudinal assessments of disease activity in patients continuing IV-golimumab for 6–9- and 9–12-months post-switch. During the follow-up periods, paired t-tests evaluated significance of mean improvements.
The study revealed the following findings:
- Most RA patients with disease activity assessments during the 6-month follow-up (N = 100; mean age: 65.3 years; 81% female; 74% white) demonstrated moderate-to-high disease activity (CDAI: 73% [38/52]) at enrollment.
- On average, patients showed significant improvement in disease activity within 6–9 months of switching; mean CDAI scores improved from 21.3 to 14.1 and were durable through 9–12 months of treatment.
- Real-world patients with moderate-to-high disease activity who switched from IV-infliximab to IV-golimumab demonstrated significant and sustained improvements post-switch as measured by the CDAI.
"Given the limited real-world data documenting efficacy of IV-infliximab-to-IV-golimumab switching, these results are clinically meaningful and may assist clinicians in providing patients with improved and sustained outcomes," the authors concluded.
Reference:
Tesser, J., Lin, I., Shiff, N.J. et al. Improvement in disease activity among patients with rheumatoid arthritis who switched from intravenous infliximab to intravenous golimumab in the ACR RISE registry. Clin Rheumatol (2022). https://doi.org/10.1007/s10067-022-06116-z
KEYWORDS: rheumatoid arthritis, golimumab, infliximab, IV, intravenous, Clinical Rheumatology, disease activity, tumor necrosis factor, John Tesser, drug tolerance
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


