- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Valaciclovir Prophylaxis Effective in Preventing Herpes Zoster in SLE Patients on Anifrolumab: Study Finds
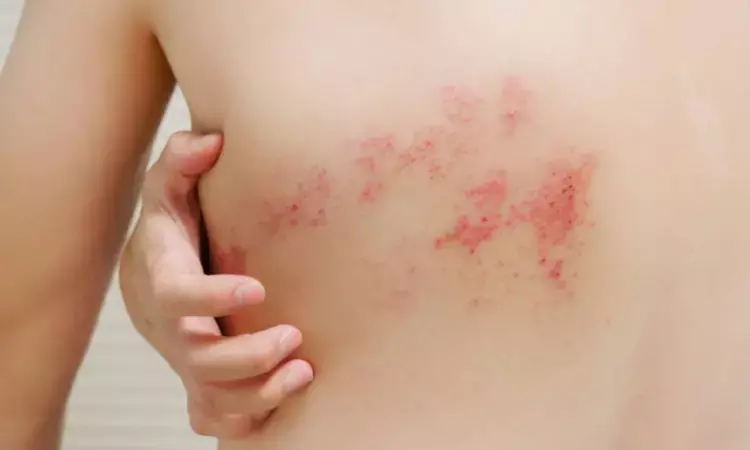
France: A recent study, published in RMD Open: Rheumatic & Musculoskeletal Diseases has confirmed valaciclovir's efficacy in preventing herpes zoster (HZ) infection in patients with systemic lupus erythematosus (SLE) receiving anifrolumab, a monoclonal antibody used for treating SLE. The study, which builds on data from earlier clinical trials, highlights the significant risk of HZ in patients undergoing anifrolumab therapy, particularly those without prophylactic measures.
"Antiviral drug valaciclovir (Valtrex), when used prophylactically, effectively prevented herpes zoster attacks in patients with systemic lupus erythematosus being treated with anifrolumab (Saphnelo), presenting a potential alternative to the zoster vaccine," the researchers reported.
Herpes zoster, commonly known as shingles, is a painful viral infection when the varicella-zoster virus reactivates, often resulting in a blistering rash. Systemic lupus erythematosus increases the risk of herpes zoster by 2.5 times compared to the general population, especially in younger patients or those on immunosuppressants or anti-interferon-alpha antibodies. Anifrolumab, a treatment for SLE, further raises the risk of HZ. In clinical trials, patients on anifrolumab had a higher incidence of HZ than those on placebo, particularly in the first year. While a recombinant HZ vaccine exists, it’s not available everywhere, and there are no clear guidelines on preventing HZ in patients using anifrolumab, including valaciclovir use as a preventive measure.
Against the above background, Ludovic Trefond, Université Clermont Auvergne, Clermont-Ferrand, France, and colleagues aimed to determine the prevalence of herpes zoster in real-world settings and explore the potential benefits of using valaciclovir as a preventive measure. The decision to include valaciclovir in treatment was made based on the discretion of both the physician and the patient.
For this purpose, the researchers conducted a multicenter observational study involving patients with SLE treated with anifrolumab for at least three months between 2021 and 2024 in internal medicine, dermatology, and rheumatology departments in France.
The study included 132 patients (92% women) with a mean age of 42.0 years. Among them, 87 (65.9%) received prophylactic valaciclovir (69 with 500 mg/day and 18 with 1000 mg/day), while 45 (34.1%) did not. Two patients had received the live attenuated vaccine, and none had received the recombinant vaccine. Thirteen patients (9.8%) had a history of HZ before starting anifrolumab.
The investigation uncovered the following findings:
- The two groups had similar demographic and clinical characteristics, including age and medication use.
- Patients on valaciclovir had a higher history of HZ (14.9% versus 0%).
- Fourteen patients discontinued anifrolumab: 10 due to ineffectiveness, 2 for pregnancy, and 2 due to infection.
- The median follow-up duration on anifrolumab was 234 days.
- Four patients in the non-valaciclovir group developed HZ at various time points after starting anifrolumab.
- HZ frequencies in the non-valaciclovir group: 2.2% at 3 months, 6.2% at 6 months, and 23% at 12 months.
- None of the valaciclovir-treated patients developed HZ.
- Univariate survival analysis showed a significantly lower risk of HZ in the valaciclovir group (HR 0.08).
- The locations of HZ in the non-valaciclovir group included the lumbar, cervical, and intercostal regions.
- Patients with HZ were treated with HCQ, prednisone, and mycophenolate mofetil alongside anifrolumab.
- None of the HZ cases were severe, and no patients required hospitalization or discontinuation of anifrolumab. One patient experienced HZ neuralgia.
"Despite its observational design and low incidence of zoster events, our study supports prior clinical trial data showing that many patients on anifrolumab without prophylaxis experience herpes zoster. It also suggests that valaciclovir effectively prevents HZ infection in SLE patients on anifrolumab. This is especially important for SLE patients who cannot access or receive the recombinant HZ vaccine," the authors concluded.
Reference:
Trefond L, Chasset F, Jachiet M, et alEfficacy of valaciclovir in preventing herpes zoster in patients receiving anifrolumabRMD Open 2025;11:e005076. doi: 10.1136/rmdopen-2024-005076
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


