- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Among infants of RDS supported by CPAP, Surfactant Therapy fails to improve outcomes
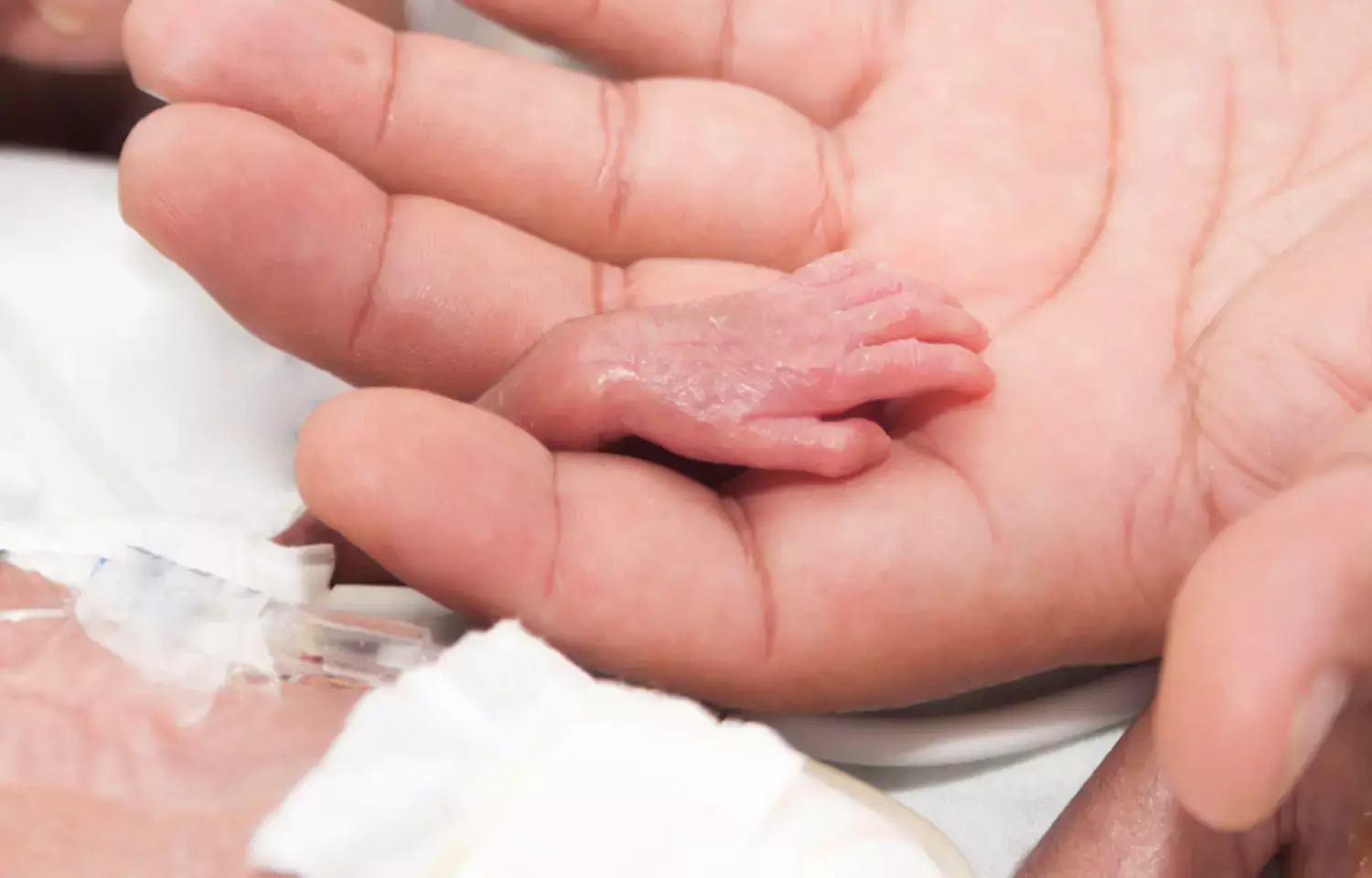
In a follow-up study of a groundbreaking randomized clinical trial, researchers have shed light on the effects of Minimally Invasive Surfactant Therapy (MIST) in preterm infants with respiratory distress syndrome. The study, conducted across 33 tertiary-level neonatal intensive care units in 11 countries, aimed to determine the impact of MIST on a crucial outcome: death or neurodevelopmental disability (NDD) at 2 years corrected age. The study results showed that MIST did not reduce the composite outcome of death or neurodevelopmental disability at 2 years of age in preterm infants supported with CPAP.
The study was published in the journal JAMA Network on September 11, 2023.
MIST involves the administration of surfactant via a thin catheter to preterm infants with respiratory distress syndrome. As there is a definitive need to assess the long-term effects of surfactant administration via a thin catheter (minimally invasive surfactant therapy [MIST]) in preterm infants with respiratory distress syndrome, researchers conducted a follow-up study of a randomized clinical trial to examine the effect of MIST on death or neurodevelopmental disability (NDD) at 2 years corrected age.
This innovative approach was evaluated in a randomized clinical trial involving 486 infants born between 25 and 28 weeks of gestation, all of whom received continuous positive airway pressure (CPAP) support. Among these infants, 242 were assigned to receive MIST, while the control group of 244 received a sham treatment. The exogenous surfactant was 200 mg/kg poractant alfa supplied via a catheter. The primary objective of the study was to assess the occurrence of death or moderate to severe NDD at 2 years of corrected age. Other outcomes that were measured were hospitalizations for respiratory illness and parent-reported wheezing or breathing difficulty in the first 2 years.
Key Findings:
- Surprisingly, the results showed that there was no significant difference in the incidence of death or NDD between the MIST group (36.3%) and the control group (36.1%).
- This outcome suggests that MIST did not reduce the risk of these adverse outcomes by the time the infants reached 2 years of age.
- Infants who received MIST exhibited lower rates of adverse respiratory outcomes during their first two years of life.
- Specifically, hospitalization due to respiratory illness occurred in 25.1% of infants in the MIST group, compared to 38.2% in the control group.
- Additionally, parent-reported wheezing or breathing difficulty was less common in the MIST group (40.6%) than in the control group (53.6%).
Thus, despite not achieving expected results regarding death or NDD, the study contributes valuable insights into the treatment of preterm infants regarding the discovery of improved respiratory outcomes among infants who received MIST. This research opens doors for further exploration and highlights the importance of innovative approaches in neonatal care.
Further reading: Dargaville PA, Kamlin COF, Orsini F, et al. Two-Year Outcomes After Minimally Invasive Surfactant Therapy in Preterm Infants: Follow-Up of the OPTIMIST-A Randomized Clinical Trial. JAMA. Published online September 11, 2023. doi:10.1001/jama.2023.15694
BDS, MDS
Dr.Niharika Harsha B (BDS,MDS) completed her BDS from Govt Dental College, Hyderabad and MDS from Dr.NTR University of health sciences(Now Kaloji Rao University). She has 4 years of private dental practice and worked for 2 years as Consultant Oral Radiologist at a Dental Imaging Centre in Hyderabad. She worked as Research Assistant and scientific writer in the development of Oral Anti cancer screening device with her seniors. She has a deep intriguing wish in writing highly engaging, captivating and informative medical content for a wider audience. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751



