- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
CPAP preferred respiratory support after extubation in critically ill children: JAMA
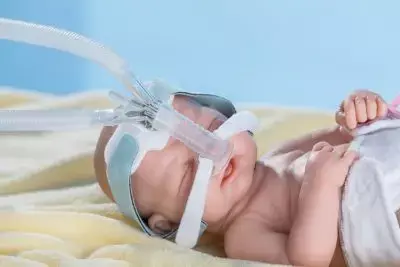
Postextubation respiratory support has been traditionally provided in the PICU setting using continuous positive airway pressure (CPAP). Recently, high-flow nasal cannula (HFNC) therapy has become a popular alternative due to ease of use, perceived greater patient comfort, and the ability to discharge children to general wards while receiving HFNC. However, in a recent study, researchers reported that HFNC failed to meet the criterion for noninferiority for time to liberation from respiratory support when compared with CPAP following extubation. The study findings were published in the JAMA on April 07, 2022.
Optimizing the first-line choice of post-extubation respiratory support should reduce extubation failures and the overall duration of invasive and non-invasive respiratory support. However, the optimal first-line mode of noninvasive respiratory support following extubation of critically ill children is not known. Therefore, Dr Padmanabhan Ramnarayan and his team conducted a study to evaluate the noninferiority of high-flow nasal cannula (HFNC) therapy as the first-line mode of noninvasive respiratory support following extubation, compared with continuous positive airway pressure (CPAP), on time to liberation from respiratory support.
In this pragmatic, multicenter, randomized, noninferiority FIRST-ABC trial, the researchers included six hundred children aged 0 to 15 years clinically assessed to require noninvasive respiratory support within 72 hours of extubation. The children were randomized to start either HFNC at a flow rate based on patient weight (n = 299) or CPAP of 7 to 8 cm H2O (n = 301). The major outcome assessed was time from randomization to liberation from respiratory support, defined as the start of a 48-hour period during which the child was free from all forms of respiratory support (invasive or noninvasive), assessed against a noninferiority margin of an adjusted hazard ratio (HR) of 0.75. They further assessed 6 other secondary outcomes, including mortality at day 180 and reintubation within 48 hours.
Key findings of the study:
- Upon analysis, the researchers found that HFNC failed to meet noninferiority, with a median time to the liberation of 50.5 hours vs 42.9 hours for CPAP (adjusted HR, 0.83).
- They observed similar results across prespecified subgroups.
- Among 6 prespecified secondary outcomes, they noted that 5 showed no significant difference, including the rate of reintubation within 48 hours (13.3% for HFNC vs 11.5 % for CPAP).
- However, they noted that mortality at day 180 was significantly higher for HFNC (5.6% vs 2.4% for CPAP; adjusted odds ratio, 3.07).
- They reported that the most common adverse events were abdominal distension (HFNC: 8/281 [2.8%] vs CPAP: 7/272 [2.6%]) and nasal/facial trauma (HFNC: 14/281 [5.0%] vs CPAP: 15/272 [5.5%]).
The authors concluded, "Among critically ill children requiring noninvasive respiratory support following extubation, HFNC compared with CPAP following extubation failed to meet the criterion for noninferiority for time to liberation from respiratory support."
In an accompanying editorial, Dr Christopher M. Horvat and his team wrote, " Higher mortality with HFNC compared with CPAP has been observed among children in other settings, although the reasons are unclear. That HFNC did not meet noninferiority criteria in the FIRST-ABC trial and potentially led to higher 180-day mortality may favor the use of CPAP until more evidence becomes available. The FIRST-ABC trial provides helpful information on preferred approaches to respiratory management in the PICU, although uncertainty still dominates clinical decision-making involving optimal respiratory support following extubation for pediatric patients."
For further information:
Keywords: critically ill children, high-flow nasal cannula, continuous positive airway pressure, respiratory support, pediatric intensive care units, infant mortality rate, reintubation rate, FIRST-ABC trial, JAMA.
Medical Dialogues Bureau consists of a team of passionate medical/scientific writers, led by doctors and healthcare researchers. Our team efforts to bring you updated and timely news about the important happenings of the medical and healthcare sector. Our editorial team can be reached at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


