- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Use of midline catheters better PIVCs for Extended Peripheral Therapy among kids: JAMA
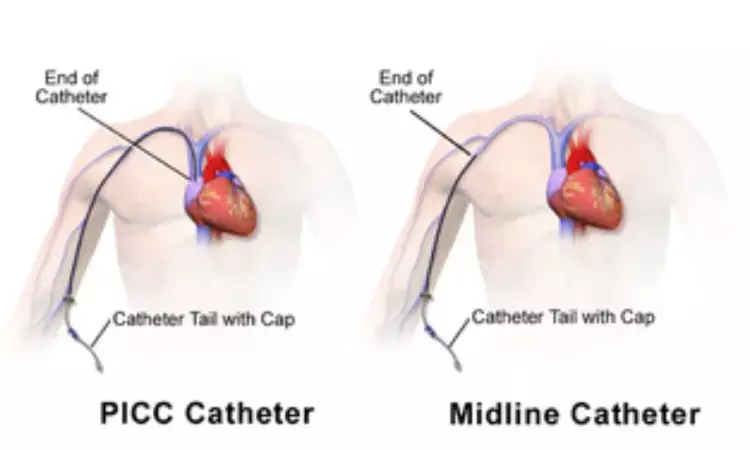
A recent study published in the Journal of the American Medical Association highlights the importance of prioritizing midline catheter (MC) insertion for patients in need of peripherally compatible intravenous therapy lasting four days or more. Midline catheters offer potential benefits in terms of longer functional use and reduced failure rates when compared to traditional Peripheral intravenous catheters (PIVCs). Therefore, Tricia Kleidon and her team conducted this study to compare the performance of MCs versus PIVCs in preventing device failure.
This pragmatic, randomized clinical trial with an embedded pilot study took place from July 2020 to May 2022 at a quaternary pediatric hospital in Brisbane, Queensland, Australia. Eligible patients were aged 1 to 18 years and required peripherally compatible intravenous therapy lasting four days or more. Patients were randomly assigned to receive either a PIVC or MC, with stratification based on age (≤5 years, >5 years). Each patient had one catheter studied.
The primary outcome measured was device failure, defined as the premature cessation of device function. Secondary outcomes included the number of insertion attempts, insertion failure, pain during insertion, procedural time, satisfaction of patients/parents with the insertion, device dwell time, complications during dwell time, the need for additional vascular access devices to complete treatment, clinician satisfaction at removal, and healthcare costs.
The key findings of this study:
Out of the 128 patients who were randomly allocated to the study groups, 127 patients underwent device insertion, with 65 (51.2%) in the PIVC group and 62 (48.8%) in the MC group.
All patients were included in the intention-to-treat analysis. The incidence of device failure was lower among patients in the MC group (10 [16.1%]) when compared to those in the PIVC group. MCs were associated with reduced insertion attempts, prolonged dwell time, and a lower proportion of patients needing additional vascular access devices to complete their treatment in both the MC group (4 [6.5%]) and PIVC group.
In comparison to PIVCs, the utilization of MCs resulted in higher levels of patient satisfaction (9.0 vs. 7.1 out of 10; P = .002) and parent satisfaction (9.1 vs. 8.2 out of 10; P = .02), as well as decreased healthcare costs (AUS −$151.67 [US −$101.13] per person; 95% credible interval, AUS −$171.45 to −$131.90 [US −$114.20 to −$87.95]).
Reference:
Kleidon, T. M., Gibson, V., Cattanach, P., Schults, J., Royle, R. H., Ware, R. S., Marsh, N., Pitt, C., Dean, A., Byrnes, J., Rickard, C. M., & Ullman, A. J. (2023). Midline Compared With Peripheral Intravenous Catheters for Therapy of 4 Days or Longer in Pediatric Patients. In JAMA Pediatrics. American Medical Association (AMA). https://doi.org/10.1001/jamapediatrics.2023.3526
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


