- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA Fast Tracks new drug combo for treating Obstructive Sleep Apnea
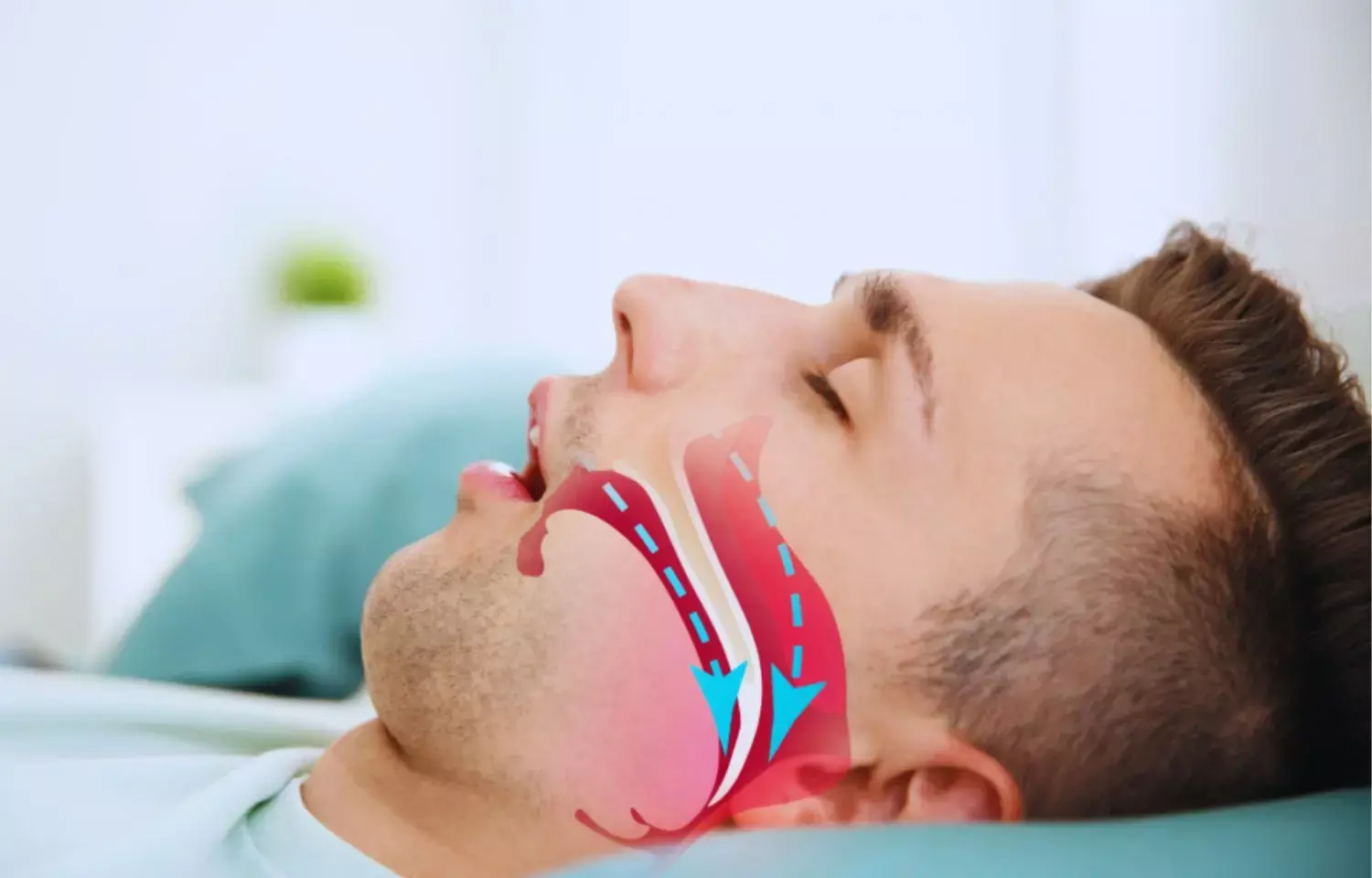
The Food and Drug Administration (FDA) has granted Fast Track designation to an investigational drug AD109 for the treatment of obstructive sleep apnea (OSA). The new oral drug is a combination of atomoxetine and aroxybutynin which works by activating the upper airway dilator muscles and maintaining an open airway during sleep.
"Fast Track designation is a significant milestone in the development of AD109 and provides an accelerated regulatory pathway that recognizes the urgent need for new pharmacologic treatments for OSA that are easier for people to tolerate," said Larry Miller, M.D., Chief Executive Officer of Apnimed. "Currently the vast majority of more than 35 million Americans who have OSA remain untreated despite the potential for serious health risks associated with the condition, including cardiovascular disease and diabetes.
We will continue to work closely with the FDA to support the development and review of AD109 beginning with the trial design for our Phase 3 program, which we anticipate initiating at the end of 2022."
FDA's Fast Track designation is intended to facilitate the development and expedite the review of new drugs to treat serious conditions and that fill an unmet medical need. The benefits of Fast Track designation include opportunities for frequent meetings with the FDA to discuss development plans, trial design, and data needed to support drug approval, as well as the ability to submit a New Drug Application (NDA) on a rolling basis, and eligibility for priority review, if relevant criteria are met.
Reference:
Apnimed granted FDA Fast Track designation for AD109, a novel first-in-class oral pharmacologic combination for the treatment of obstructive sleep apnea (OSA). News release. Apnimed. Accessed June 28, 2022. https://www.businesswire.com/news/home/20220628005075/en/Apnimed-Granted-FDA-Fast-Track-Designation-for-AD109-a-Novel-First-in-Class-Oral-Pharmacologic-Combination-for-the-Treatment-of-Obstructive-Sleep-Apnea-OSA
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


