- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
intranasal corticosteroids not recommended for most children with OSA: study
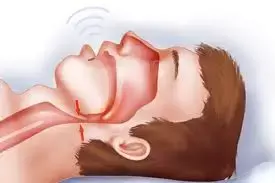
According to a recent study published in The Chest, treatment with intranasal corticosteroids is ineffective in children with obstructive sleep apnea syndrome.
Obstructive sleep apnea occurs when the muscles in your throat that support soft tissues such as the tongue and soft palate temporarily relax. When these muscles relax, your airway narrows or closes and breathing is momentarily interrupted.
Intranasal corticosteroids (INCS) are often used to treat obstructive sleep apnea syndrome (OSAS) in children. However, their effectiveness has not been rigorously tested.
In this randomised, double-blind, placebo-controlled study, children aged 5 to 12 years (n=134) with obstructive sleep apnea syndrome were randomised 2:1 to 3 months of intranasal corticosteroids or placebo. Children in the Intranasal corticosteroids arm were then re-randomized to 9 months of Intranasal corticosteroids or placebo. Polysomnography, symptoms, and neurobehavioral findings were measured at baseline, 3, and 12 months. The primary outcome was the obstructive apnea-hypopnea index (OAHI) change at 3 months, available for 122 children. The secondary outcome was the obstructive apnea-hypopnea index change at 12 months, available for 70 children.
Results of the study:
• The median [IQR] age and obstructive apnea-hypopnea index at baseline for the entire group were 7.9 years and 5.8/hr.
• Obstructive apnea-hypopnea index changes at 3 and 12 months were not different between the 2 groups.
• Obstructive sleep apnea syndrome symptoms and neurobehavioral outcomes were not different between the Intranasal corticosteroids and placebo groups at 3 and 12 months.
• Obstructive apnea-hypopnea index decreased significantly from 7.2 to 3.7/hour in 38 children who received intranasal corticosteroids for 12 months.
Thus, Tapia et al. concluded that in children with obstructive sleep apnea syndrome, treatment with intranasal corticosteroids did not result in significant polysomnographic, neurobehavioral, and symptom changes at 3 and 12 months of treatment. 12 months of intranasal corticosteroid treatment resulted in a statistically significant but not clinically significant decrease in the obstructive apnea-hypopnea index.
Reference:
Ignacio E. Tapia et al. A trial of intranasal corticosteroids to treat the childhood obstructive sleep apnea syndrome. June 29, 2022DOI:https://doi.org/10.1016/j.chest.2022.06.026
Keywords:
Intranasal, corticosteroids, children, obstructive, sleep, apnea, Randomized Controlled Trial, Double-Blind Method, Intention, Treat, Analysis, Fluticasone, Ignacio E. Tapia, Justine Shults, Christopher M. Cielo, Andrea B. Kelly, Lisa M. Elden, Jonathan M. Spergel, Ruth M. Bradford, Mary Anne Cornaglia, Laura M. Sterni, Jerilynn Radcliffe, CHEST journal
Dr. Shravani Dali has completed her BDS from Pravara institute of medical sciences, loni. Following which she extensively worked in the healthcare sector for 2+ years. She has been actively involved in writing blogs in field of health and wellness. Currently she is pursuing her Masters of public health-health administration from Tata institute of social sciences. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


