- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Microbion to develop pravibismane for cystic fibrosis-related lung infections
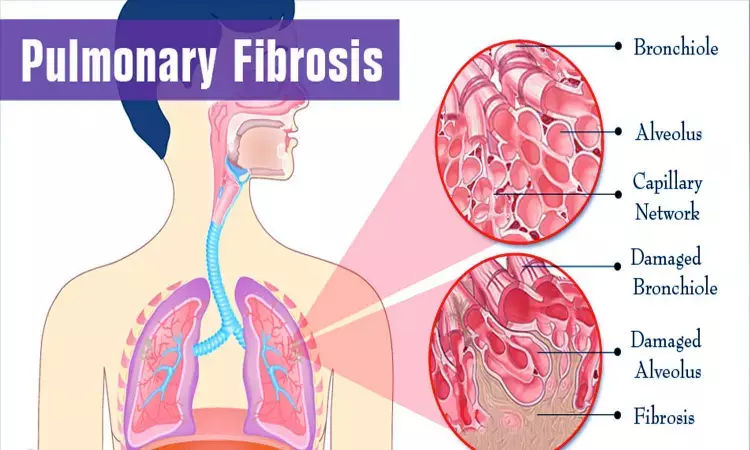
BOSTON – Microbion is scaling up its efforts pto develop its anti-infective drug pravibismane for the treatment of cystic fibrosis (CF)-related pulmonary infections.
The research project is CARB-X's first award for the development of an inhaled antibiotic to treat chronic lung infections and potentially infections in people living with CF.
"Microbion's new class of anti-infective represents a novel drug that has shown potent activity against antibiotic-resistant bacterial pathogens and the biofilms these pathogens produce. Microbion's pravibismane, if successful and eventually approved for use in patients, has the potential to be a critical new weapon in the fight against chronic and resistant infections," said Erin Duffy, Chief of Research and Development of CARB-X.
The funding from CARB-X, along with a separate award announced today of up to $5.6 million from the Cystic Fibrosis Foundation, will help Microbion further explore the potential of pravibismane to kill drug-resistant bacteria and their biofilms and test its safety as an inhaled treatment for people with CF. If the results are positive, the anti-infective could next move into clinical development to test its effectiveness.
People with CF are susceptible to chronic infections due to abnormally thick, sticky mucus in their lungs which traps bacteria in the airways. These bacteria often form colonies surrounded by a protective outer layer known as biofilms, a community of bacteria that adheres to surfaces and is able to cause chronic infections. Biofilms tend to allow bacteria to both evade the immune system and become more resistant to antibiotics. At least 80 percent of infections involve bacteria that have formed biofilms, according to the National Institutes of Health, making biofilms an important target when fighting hard-to-treat infections.
Karim Lalji, Microbion's Chairman, said: "Beyond their investment, these organizations' expertise in antibiotic-resistant infections, particularly those associated with CF, will prove valuable to advancing our technology as part of a much-needed solution to address the chronic and intractable infections that are a hallmark of CF."
In laboratory tests, Microbion's drug candidate pravibismane was shown to kill drug-resistant bacteria and their biofilms, including those that pose a higher risk for people with CF such as multi-drug resistant Pseudomonas aeruginosa and nontuberculous mycobacteria (NTM).
The US Food and Drug Administration (FDA) has given the project Orphan Drug Designation, as well as Qualified Infectious Disease Product (QIDP) and Fast Track designations for inhaled pravibismane for the treatment (management) of pulmonary infections in patients with cystic fibrosis. Cystic fibrosis lung disease, estimated to affect more than 30,000 in the US and 70,000 worldwide, is characterized by infection and severe inflammation that lead to progressive deterioration in lung function. The disease represents a major health-care burden, responsible for reduced quality of life, morbidity, and premature mortality.
Supporting innovation to address the global superbug crisis
The CARB-X portfolio is the world's largest and most diverse antibacterial development portfolio with 40 active projects – antibiotics, vaccines, rapid diagnostics and other life-saving products – in five countries focused on addressing the global rise of drug-resistant bacteria.
CARB-X, led by Boston University and funded by a global partnership, is investing up to $500 million between 2016-2021 to support the development of new antibiotics, rapid diagnostics, vaccines and other life-saving products that address drug-resistant bacteria. The goal is to support projects through the early phases of development – from hit-to-lead through Phase 1 for therapeutics – so that they will attract additional private or public support for further clinical development and approval for use in patients.
Since its launch in 2016, CARB-X has announced 62 awards exceeding $235.7 million, with the potential of additional funds if project milestones are met, to accelerate the development of antibacterial products. These funds are in addition to investments made by the companies themselves. The CARB-X pipeline will continuously evolve, as projects progress and others fail for a variety of reasons.
The CF Foundation's commitment to antibiotic development
In 2018, the CF Foundation dedicated $100 million through 2023 to their Infection Research Initiative as part of a sweeping effort to advance infection research. Currently, the CF Foundation is funding 12 new industry programs to develop treatments for CF-related infections.
This news release is supported by the Cooperative Agreement Number IDSEP160030 from ASPR/BARDA and by award from Wellcome Trust and Germany's Federal Ministry of Education and Research (BMBF). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the HHS Office of the Assistant Secretary for Preparedness and Response, or other CARB-X funders.
Hina Zahid Joined Medical Dialogue in 2017 with a passion to work as a Reporter. She coordinates with various national and international journals and association and covers all the stories related to Medical guidelines, Medical Journals, rare medical surgeries as well as all the updates in the medical field. Email: editorial@medicaldialogues.in. Contact no. 011-43720751
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


