- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Triple Therapy Improves Glucose Control in Cystic Fibrosis: Study
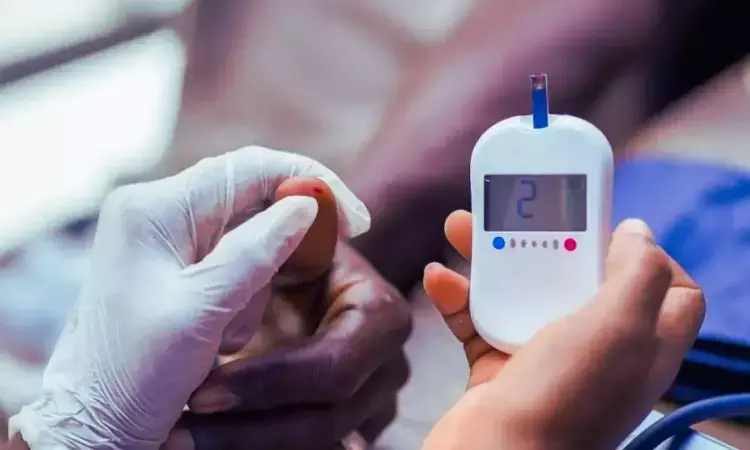
A new study published in the American Journal of Respiratory and Critical Care Medicine found that the combination of elexacaftor, tezacaftor, and ivacaftor significantly improved blood glucose regulation in cystic fibrosis patients with abnormal glucose tolerance. Over 48 weeks, patients showed reductions in 2-hour blood glucose and A1C levels, with no safety concerns.
Multiple organs are impacted by the hereditary disease known as cystic fibrosis (CF), with the pancreas and lungs being especially susceptible. Abnormal glucose metabolism, which includes reduced glucose tolerance and cystic fibrosis-related diabetes (CFRD), is a frequent consequence of CF that has a substantial influence on the health and prognosis of patients. Treatment has been transformed by the advent of cystic fbrosis transmembrane conductance regulator (CFTR) modulator medications, especially the triple combination of Ivacaftor, Tezacaftor, and Elexacaftor, which improves lung function and slows the course of the illness.
Recent data indicates that these treatments could possibly affect glucose control and pancreatic function. It is crucial to comprehend how Elexacaftor, Tezacaftor, and Ivacaftor affect glucose tolerance since better glycemic management can improve nutritional status, lower complications, and improve CF patients' quality of life. Thus, this research assessed how Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) affected glucose tolerance in CF patients with IGT or CFRD.
The participants who were ≥12 years old, heterozygous for F508del and a minimal function CFTR mutation, and who had either IGT or CFRD were given ELX/TEZ/IVA for 48 weeks as part of this trial. ELX/TEZ/IVA was administered to 69 volunteers. Blood glucose levels after a 2-hour oral glucose tolerance test (OGTT) were the main outcome.
The individuals' mean change was -35.0 mg/dL (95%CI:-49.2,-20.7; P<0.0001) (-1.9 mmol/L [95%CI: 2.7, 1.2]) from baseline to the average of Weeks 36 and 48. The percentage of patients who improved in their dysglycemia classification (CFRD, IGT, and normal glucose tolerance) at Week 48 and safety were secondary objectives.
At Week 48, dysglycemia classification improved for 37.7% (95%CI:24.8,52.1) of participants with impaired glucose tolerance at baseline. When compared to 13.0% at baseline, 35.5% of patients had normal glucose tolerance by Week 48. Safety was in line with ELX/TEZ/IVA's defined safety profile.
Overall, important within-group reductions in blood glucose levels after OGTT and better dysglycemia classification in CF patients with early IGT or CFRD were among the clinically important improvements in blood glucose management brought about by ELX/TEZ/IVA medication.
Source:
Durieu, I., Clements, B., Fabrizzi, B., Mall, M. A., McKone, E., Ramsey, B., Tullis, E., Taylor-Cousar, J. L., van der Meer, R., Bachman, E., Chin, A., Conner, S., Jennings, M., Weinstock, T., Colombo, C., & Robinson, P. (2025). Impact of elexacaftor/tezacaftor/ivacaftor on glucose tolerance and abnormal glucose metabolism: A phase 3b, open-label clinical trial. American Journal of Respiratory and Critical Care Medicine. https://doi.org/10.1164/rccm.202411-2312OC
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


