- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
ICMR approves Odisha made COVID rapid antigen test kit
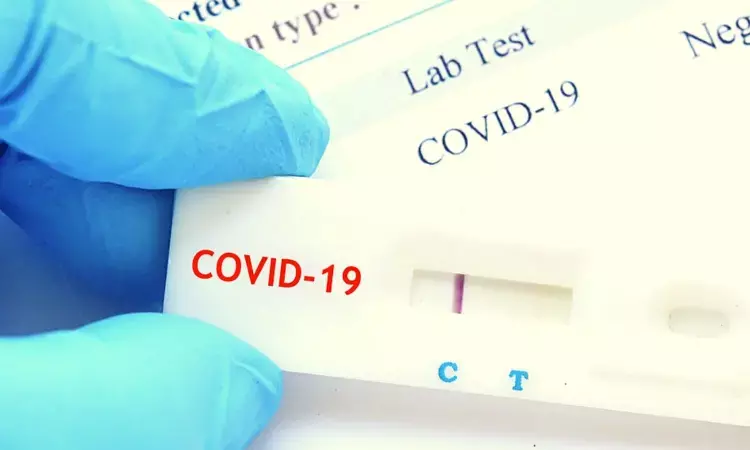
The ICMR has approved the test kits based on the RMRC's internal validation and a third party's (an expert group from Kerala) report on efficacy and accuracy.
Bhubaneswar: The Indian Council of Medical Research (ICMR) has validated the COVID-19 rapid antigen test kit developed by a private company in Odisha.
The kit has been developed by IMGENEX India Pvt Ltd, Bhubaneswar with intellectual input from Regional Medical Research Centre (RMRC). The kit can detect various variants of COVID-19 by using nasopharyngeal swab samples.
Also Read:Abbott Labs unit recalls 2 COVID lab test kits over false positive results
RMRC director Dr Sanghamitra Pati said: "The IMGENEX India Pvt Ltd, Bhubaneswar first started developing the kit and RMRC Bhubaneswar provided intellectual support for in-house testing and validation. Subsequently, we submitted the proposal for approval." She said the kit ImCOV-Ag has very high sensitivity and specificity and can detect all variants of COVID-19.
The ICMR has approved the test kits based on the RMRC's internal validation and a third party's (an expert group from Kerala) report on efficacy and accuracy.
IMGENEX, CEO, Dr Sujay Singh said they started work on the kit in June 2021 and ICMR has approved it on Thursday following certain procedures.
He said with existing facilities, the firm can manufacture 2 lakh kits which can be ramped up to 20 lakh kits per month. Singh said that the kit is likely to be available in the market in two months.
Since it is the first such kit in eastern India, the states in this region can easily avail the facility. Singh said the market price of the kit will be lower than others now available in the market.
The ICMR, has so far validated as many as 150 Antigen based Rapid Test Kits (including 31 revalidations), sources in the health department said.
Also Read:Delhi caps COVID-19 RT-PCR test price in private hospitals, labs at Rs 300
Medical Dialogues Bureau consists of a team of passionate medical/scientific writers, led by doctors and healthcare researchers. Our team efforts to bring you updated and timely news about the important happenings of the medical and healthcare sector. Our editorial team can be reached at editorial@medicaldialogues.in.


