- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
USFDA approves a prosthesis (OPRA) for over the knee amputees
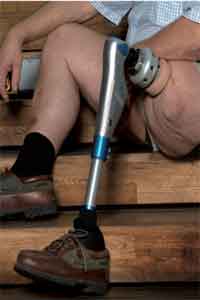
The U.S. Food and Drug Administration has authorized use of the first prosthesis marketed in the U.S. for adults who have amputations above the knee and who have rehabilitation problems with, or cannot use, a conventional socket prosthesis.
The Osseoanchored Prostheses for the Rehabilitation of Amputees (OPRA) device instead uses fixtures and screws implanted into the patient's remaining thigh bone to connect an external prosthetic limb.
A conventional leg prosthesis uses a specially-fitted cup-like shell called a socket that fits over the remaining portion of the patient's leg (the residual limb) to secure the device to the leg. Some patients may not have a long enough residual limb to properly fit a socket prosthesis or may have other conditions, such as scarring, pain, recurrent skin infections, or fluctuations in the shape of the residual limb that prevent them from being able to use a prosthesis with a socket.
"Prostheses can help people who have lost a leg due to trauma or cancer to regain mobility and to more easily participate in everyday activities," said William Maisel, M.D., M.P.H., acting director of the Office of Device Evaluation in the FDA's Center for Devices and Radiological Health. "The OPRA device may help those with above-the-knee amputations who have had problems with rehabilitation and have not been able to benefit from available socket prostheses."
The OPRA device is installed with two surgical procedures. In the first procedure, a cylinder-shaped fixture is implanted into the central canal of the remaining thigh bone. Approximately six months later, after tissue has grown to anchor the fixture and the skin tissue has healed, a second surgery is performed to implant a rod that attaches to the fixture from the previous surgery. This rod extends through the skin at the bottom of the patient's residual limb and connects to the prosthetic leg.
After the second surgery, the patient works with a trained physical therapist to gradually place weight on the OPRA device using a training prosthesis. Patients require about six months of training and rehabilitation after the second surgery, before being fitted with their own customized prosthesis by a trained prosthetist.
The OPRA device received a Humanitarian Use Device (HUD) designation and was reviewed through the Humanitarian Device Exemption (HDE) pathway. An HDE is an application that is similar to a premarket approval application (PMA) but it is exempt from the effectiveness requirements that apply to PMAs. Devices are eligible for HUD designation if they are designed to treat or diagnose a disease or condition that affects or is manifested in fewer than 4,000 individuals in the U.S. per year. In order to receive HDE approval for a HUD, a company must demonstrate safety and probable benefit of the device (i.e., that the device will not expose patients to an unreasonable or significant risk of illness or injury, and that the probable benefit of the device outweighs the risk of injury or illness from its use), and that there are no legally marketed comparable devices, other than a device approved under the HDE or investigational device exemption (IDE), available to treat or diagnose the disease or condition.
Data supporting the safety and probable benefit of the OPRA device included mechanical testing of the device's parts when subjected to weight, twisting, bending and simulated repeated use, and a two-year, 51-subject clinical trial. The clinical trial found that study subjects reported increased prosthetic use, and improved mobility, comfort, function, and quality of life compared to the subjects' own outcomes prior to the surgeries. The most common adverse event was infection.
The OPRA device is manufactured by Integrum AB in Molndal, Sweden.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation's food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Meghna A Singhania is the founder and Editor-in-Chief at Medical Dialogues. An Economics graduate from Delhi University and a post graduate from London School of Economics and Political Science, her key research interest lies in health economics, and policy making in health and medical sector in the country. She is a member of the Association of Healthcare Journalists. She can be contacted at meghna@medicaldialogues.in. Contact no. 011-43720751


