- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Cerebral protection devices may not reduce risk of clinical stroke post TAVR, NEJM study
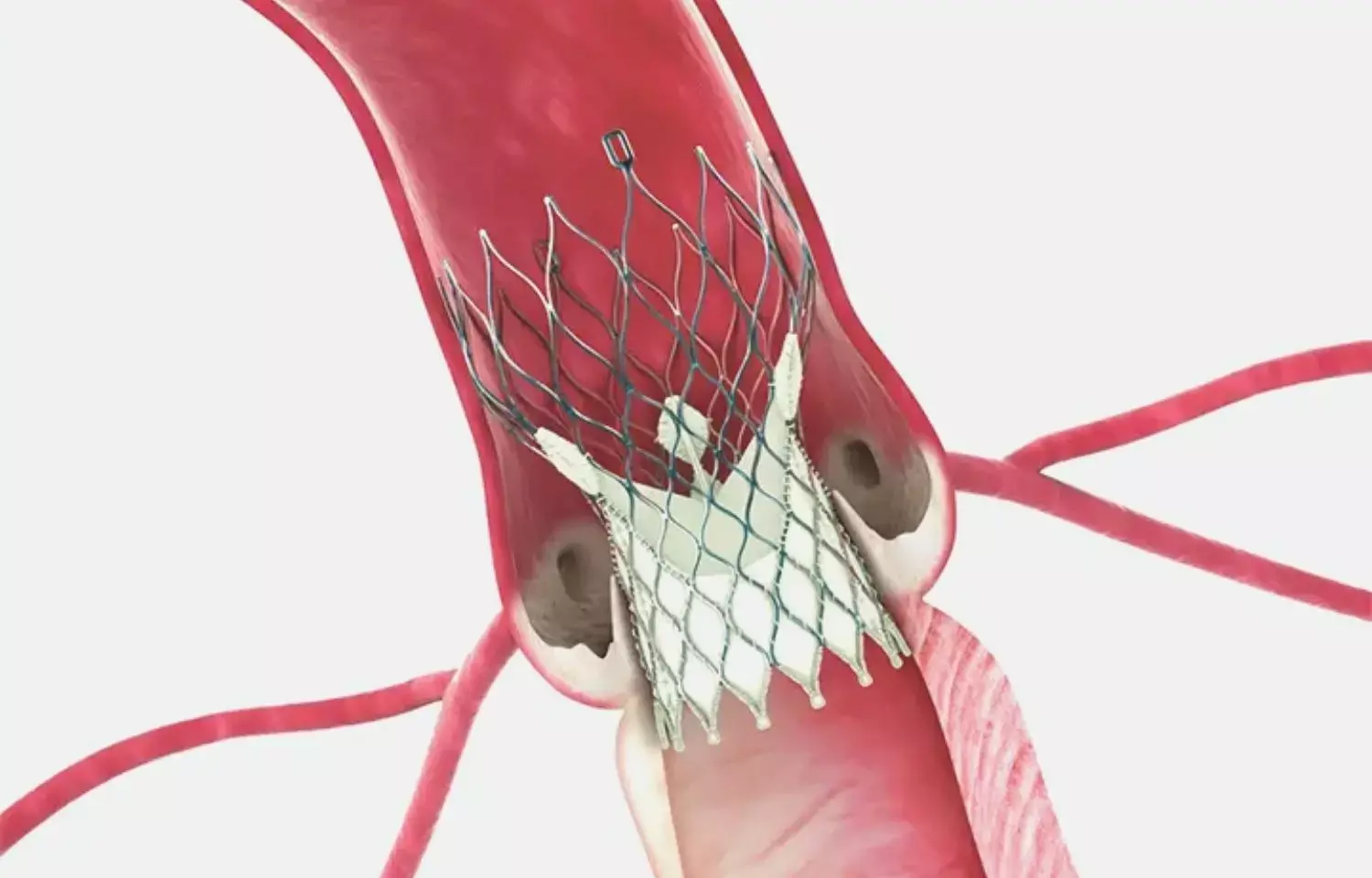
It is well known that TAVR for the treatment of aortic stenosis can lead to embolization of debris. Capture of these debris by cerebral embolic protection (CEP) devices may thus hypothetically reduce the risk of stroke. However much of the evidence available for FDA approved Sentinel CEP device is generated from trials that had MRI based ischemic lesions as primary endpoints rather than clinical stroke which is practically more relevant.
In this regard, The PROTECTED TAVR trial was conducted with clinical stroke as primary endpoint and it was found that the use of CEP did not have a significant effect on the incidence of periprocedural stroke. The trial results were published this week in NEJM.
Even in the absence of clinical symptoms, the majority of patients (68 to 93%) who have undergone TAVR have defects identified on diffusion-weighted perfusion imaging. In the present trial, authors randomly assigned patients with AS in a 1:1 ratio to undergo transfemoral TAVR with CEP (CEP group) or without CEP (control group).
The primary end point was stroke within 72 hours after TAVR or before discharge (whichever came first) in the intention-to-treat population. Disabling stroke, death, transient ischemic attack, delirium, major or minor vascular complications at the CEP access site, and acute kidney injury were also assessed. A neurology professional examined all the patients at baseline and after TAVR.
It was found that the difference in the incidence of stroke between the CEP group and the control group was not significant (2.3% vs. 2.9%). A disabling stroke, one of 15 secondary end points, occurred in 0.5% in the CEP group and in 1.3% in the control group. The number needed to treat to prevent one disabling stroke was 125 patients.
"The fact that the negative results of the study are in part due to the low incidence of stroke events in the control group conveys good news — stroke frequency without CEP is low in contemporary practice as compared with the early experience with TAVR and is likely due to operator experience, technical refinements to procedural equipment, and the use of TAVR in healthier patients", observed Caroll et al in an accompanying editorial.
How will clinicians respond to the PROTECTED TAVR trial?
The results from the current trial may change the use of the Sentinel CEP device in practice. Until now, the clinicians who use the device have been influenced by seeing retrieved debris with the expectation of an associated clinical benefit, which has not been confirmed by the primary end-point results of the current trial.
Some may continue to use the Sentinel device because of its safety and the suggestion that it prevents disabling strokes. Other clinicians and health care systems could balk at the prospect of adding a major cost to the TAVR procedure without a proven clinical benefit.
As a whole, this trial strikes a serious blow to the hope that this first CEP device would have a definitive effect in the prevention of TAVR-related strokes. The "smoking gun" of captured debris was not associated with high-quality evidence of stroke prevention.
Source: NEJM:
1. DOI: 10.1056/NEJMoa2204961
2. DOI: 10.1056/NEJMe2210185
MBBS, MD , DM Cardiology
Dr Abhimanyu Uppal completed his M. B. B. S and M. D. in internal medicine from the SMS Medical College in Jaipur. He got selected for D. M. Cardiology course in the prestigious G. B. Pant Institute, New Delhi in 2017. After completing his D. M. Degree he continues to work as Post DM senior resident in G. B. pant hospital. He is actively involved in various research activities of the department and has assisted and performed a multitude of cardiac procedures under the guidance of esteemed faculty of this Institute. He can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


