- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Change in percent atheroma volume could be surrogate marker for adverse CV events: JAMA
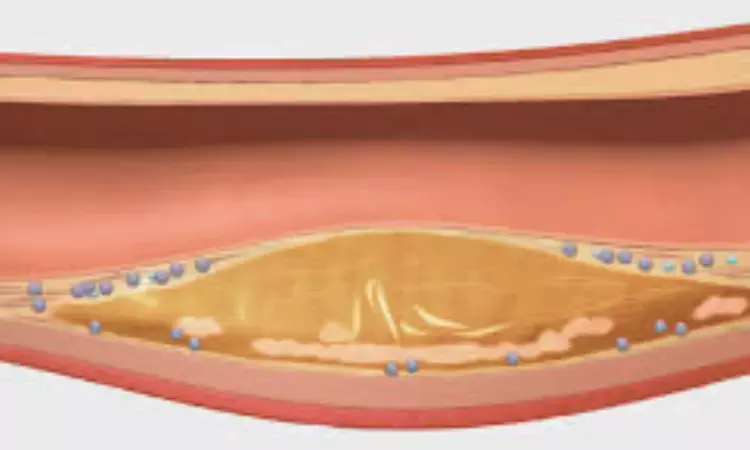
Canada: In a comprehensive meta-analysis published in JAMA Cardiology by Iulia Iatan and colleagues related to lipid-lowering therapy trials, a 1% decrease in atherosclerotic plaque volume was found to be associated with a significant 25% reduction in the odds of major adverse cardiovascular events (MACE). These findings suggest that measuring the change in plaque volume could serve as a surrogate marker for predicting cardiovascular outcomes.
The study incorporated data from 23 trials conducted between July 2001 and July 2022, involving a total of 7,407 patients. These trials assessed the effects of high-intensity lipid-lowering therapies (LLTs) on plaque regression using intravascular ultrasound (IVUS) and reported concurrent MACE outcomes. The study duration varied from 11 to 104 weeks, with patients' mean ages ranging from 55.8 to 70.2 years.
1% Plaque Reduction Equals 25% Lower MACE Risk: The analysis revealed a significant association between a 1% reduction in percent atheroma volume (PAV) and a 25% decrease in the odds of experiencing MACE.
Adjustment for Clinical Covariates: Even after adjusting for clinical covariates such as baseline PAV, age, study duration, and baseline LDL-C level, the correlation between plaque regression and reduced MACE risk remained significant. A 1% reduction in PAV was associated with a 14% lower risk of MACE.
Additional CV Risk Factors: When further adjusting for cardiovascular risk factors, the risk of MACE was reduced by 19% for each 1% decrease in PAV.
Comparison Across Treatment Arms: The meta-analysis also explored the impact of PAV change between intervention and comparator arms within studies. A 1% reduction in PAV in the intervention arm compared to the comparator arm was associated with a significant 25% reduction in MACE risk.
Overall, these results support the notion that tracking changes in atherosclerotic plaque volume, measured via IVUS in LLT trials, can serve as a valuable surrogate marker for predicting the risk of major adverse cardiovascular events.
This meta-analysis adds to the growing body of evidence demonstrating the link between plaque regression and reduced cardiovascular events. The study suggests that assessing changes in plaque volume could be a valuable tool in determining the effectiveness of new therapeutic approaches for atherosclerotic cardiovascular disease (ASCVD).
This methodology could help researchers and clinicians make informed decisions about the cost-effectiveness and value of large randomised clinical trials, offering a potential shortcut for evaluating the outcomes, safety, and adverse events associated with novel drug therapies in ASCVD management. However, given the heterogeneity in outcomes observed in this analysis, further research and data are warranted to strengthen these findings.
Reference:
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


