- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
New Insights from the AMIKINHAL Trial: Advancing Prevention Strategies for Ventilator-Associated Pneumonia
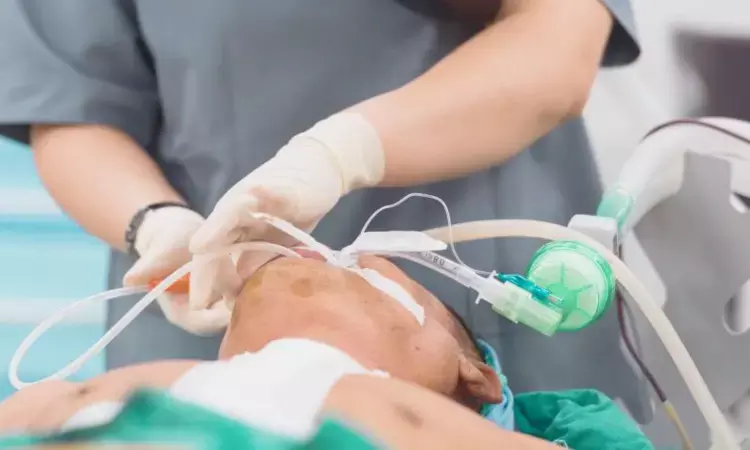
USA: A recent editorial published in the Journal of Cardiothoracic and Vascular Anesthesia offers valuable insights into the AMIKINHAL Trial and provides an update on strategies for preventing ventilator-associated pneumonia (VAP).
Ventilator-associated pneumonia remains a significant challenge in critical care settings, affecting an estimated 5% to 40% of patients on mechanical ventilation for more than 48 hours. The incidence of VAP can vary widely based on factors, including geographic location, patient demographics, and diagnostic criteria. The condition is often caused by a range of pathogens, including methicillin-sensitive Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumoniae, Escherichia coli, Serratia spp., Klebsiella pneumoniae, Pseudomonas aeruginosa, and Proteus spp.
The emergence of multidrug-resistant organisms complicates the clinical landscape, especially in certain patient populations with specific risk factors. VAP is linked to numerous negative outcomes, including increased hospital costs, extended lengths of stay, longer durations of mechanical ventilation, and elevated mortality rates. Additionally, inappropriate or excessive antimicrobial use can lead to further complications, including adverse effects and increased resistance.
In light of these challenges, the AMIKINHAL trial offers promising insights into the prevention of VAP. The clinical study investigates the efficacy of inhaled aminoglycosides in preventing the onset of VAP in mechanically ventilated patients. By targeting pathogens directly in the lungs, inhaled treatments aim to reduce the incidence of VAP and its associated complications.
According to the article, ventilator-associated pneumonia remains a critical challenge in intensive care units, impacting a significant number of patients on mechanical ventilation. In response to this issue, preventive “bundles” of interventions have been developed over the past two decades. Initially introduced by the Institute for Healthcare Improvement (IHI), these bundles included head-of-bed elevation, stress ulcer prophylaxis, and sedation interruptions. The Intensive Care Society (ICS) later expanded on these interventions by incorporating evidence-based practices such as daily spontaneous awakening trials (SATs) and spontaneous breathing trials (SBTs), which have shown promise in reducing VAP rates.
A significant development in VAP prevention is the use of inhaled antimicrobials. The AMIKINHAL trial explored the effectiveness of inhaled amikacin in preventing VAP. The multicenter, double-blind trial, conducted across 19 French ICUs, involved patients undergoing mechanical ventilation for at least 72 hours. Results indicated a lower incidence of VAP in patients receiving amikacin compared to placebo (15% versus 22%). While these findings are encouraging, the limitations of the trial included a potential overestimation of VAP incidence due to sensitive diagnostic criteria.
The AMIKINHAL trial highlights the potential of inhaled antibiotics to deliver high drug concentrations directly to the lungs, potentially reducing bacterial burden without the systemic side effects commonly associated with traditional antibiotics. However, the number needed to treat (NNT) suggests that broader adoption may be limited, especially in settings with lower VAP rates.
"Future research should refine VAP diagnostic criteria and explore alternative antibiotics and innovative nonpharmacologic interventions. Recent evidence points to the effectiveness of selective digestive decontamination (SDD) and early mobilization, which may offer additional strategies for reducing VAP incidence. Overall, the AMIKINHAL trial serves as a vital step forward in the ongoing effort to enhance VAP prevention and improve patient outcomes in critical care settings," the article concluded.
Reference:
Paul J, Patel M, Moitra V. The AMIKINHAL Trial and an Update on Prevention of Ventilator-Associated Pneumonia. J Cardiothorac Vasc Anesth. 2024 Oct;38(10):2163-2165. doi: 10.1053/j.jvca.2024.07.011. Epub 2024 Jul 11. PMID: 39107219.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


