- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Brodalumab safe option for nail psoriasis treatment: Study
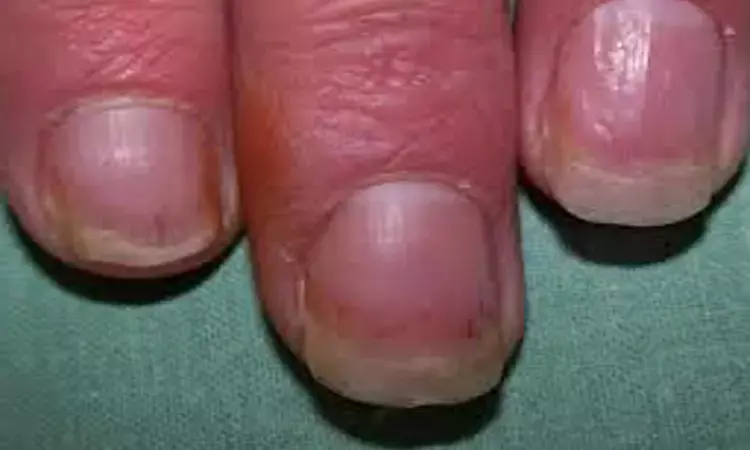
Courtesy- Journal of American Academy of Dermatology
Athens, Greece: The administration of brodalumab significantly improves nail psoriasis, finds a recent study in the Journal of the European Academy of Dermatology and Venereology.
Nail involvement is common among psoriasis patients that may significantly impair quality of life (QoL). Still, nail psoriasis treatment is largely not standardized. Brodalumab is a recombinant, fully human, anti-interleukin-17 receptor A, monoclonal antibody that exhibits long-term efficacy in plaque psoriasis due to concurrent inhibition of proinflammatory cytokines IL-17A, IL-17C, IL-17E, IL-17F and IL-17A/F heterodimer. A. Tsiogka, National and Kapodistrian University of Athens, Athens, Greece, and colleagues aimed to evaluate its efficacy in nail psoriasis as assessed by the Nail Psoriasis Severity Index (NAPSI) and the Dermatology Life Quality Index (DLQI) in a open-label, unblinded study.
For the purpose, the researchers gave subcutaneous injections of brodalumab 210 mg at baseline, weeks 1, 2, 3, and every 2 weeks thereafter to dermatology patients at the Andreas Sygos Hospital in Athens, Greece with confirmed severe plaque-type psoriasis and evident psoriatic nail disease. Nails of the patients were assessed at baseline and weeks 12 and 24.
30 patients were enrolled in the study.
Key findings of the study include:
- The mean NAPSI score for fingernails at baseline was 19.6 (standard deviation [SD], 9.3).
- At week 12 it was 9.6 (SD, 5.3) and at week 24 it was 2.63 (SD, 2.3).
- The mean NAPSI score for toenails was 24.9 (SD, 13.6) at baseline. At week 12 it was 16.1 (SD, 9.7) and at week 24 it was 7.2 (SD, 6.1).
- The mean DLQI score for finger and toenails was 22.8 (SD, 4.4) at baseline and 3.7 (SD, 2.2) at week 24.
- There was improvement in 57% (n=17) of patients with a fingernail NAPSI score of 50 by week 12 and in 100% (n=30) of patients by week 24.
- There was improvement in 23% (n=7) patients with a toenail NAPSI score of 50 by week 12 and in 97% (n=29) patients by week 24.
- The only adverse events recorded were headache in 2 patients and diarrhea in 1 patient, all of which resolved spontaneously in a few days.
The study showed brodalumab's rapid onset, high efficacy, and safety for nail psoriasis.
"Our results support the need of prolonged therapy before evaluating the full efficacy of biologics in psoriasis of this specific body site," concluded the authors.
The study titled, "Treatment of nail psoriasis with brodalumab: an open‐label unblinded study," is published in the Journal of the European Academy of Dermatology and Venereology.
DOI: https://onlinelibrary.wiley.com/doi/abs/10.1111/jdv.17055
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


