- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Crisaborole may significantly improve stasis dermatitis, finds innovative study
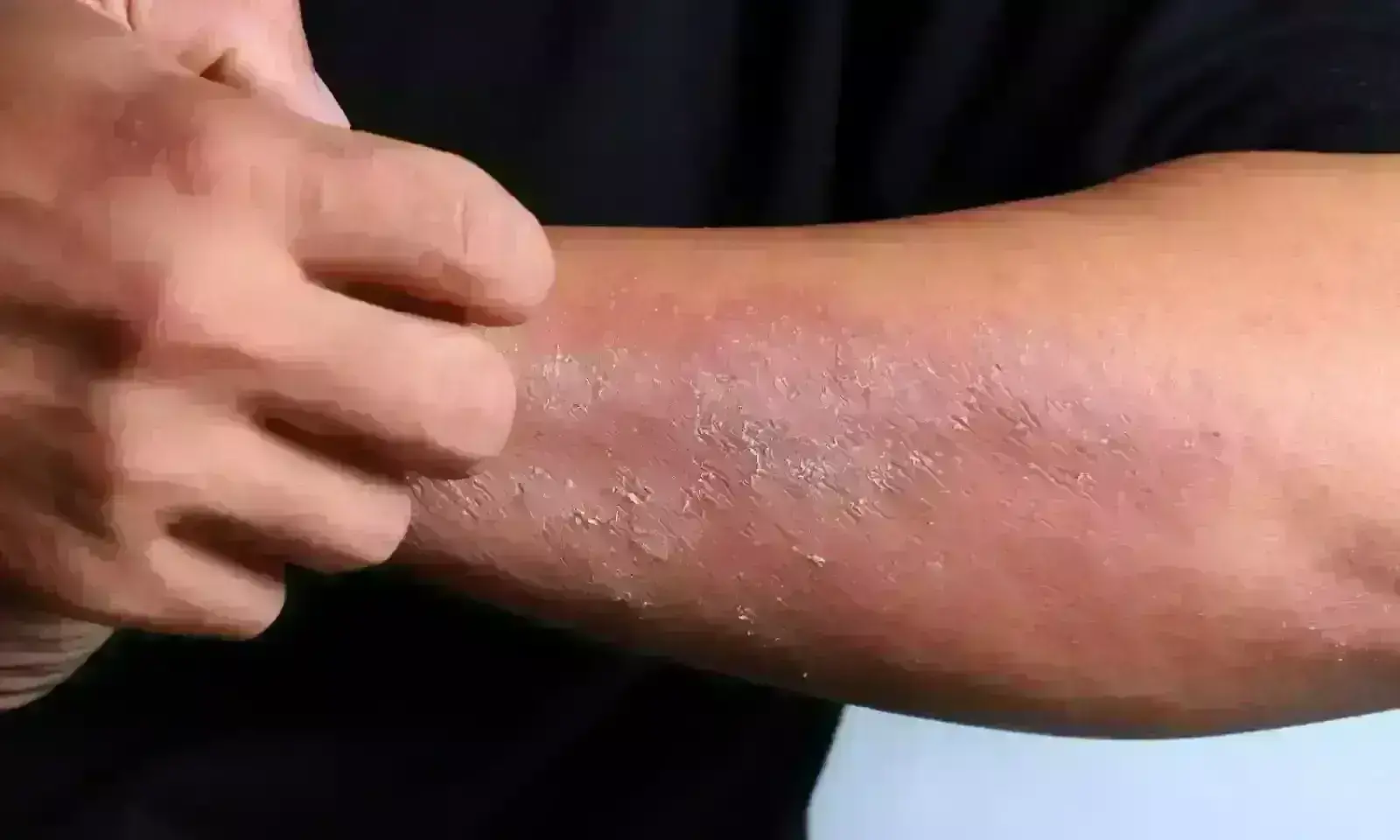
Crisaborole may significantly improve stasis dermatitis, finds aninnovative study published in the Journal of the American Academy of Dermatology.
Crisaborole ointment, 2%, is a nonsteroidal topical phosphodiesterase 4 inhibitor approved for the treatment of mild-to-moderate atopic dermatitis. A study was done to evaluate the efficacy and safety of crisaborole in stasis dermatitis (SD). In this randomized, double-blind, vehicle-controlled, decentralized phase 2a study (NCT04091087), 65 participants aged ≥45 years with SD without active ulceration received crisaborole or vehicle (1:1) twice-daily for 6 weeks. The primary end point was percentage change from baseline in total sign score at week 6 based on in-person assessment.
Results: Crisaborole-treated participants had significantly reduced total sign score from baseline versus vehicle based on in-person (nondermatologist) assessment (−32.4% vs −18.1%, P = .0299) and central reader (dermatologists) assessment of photographs (−52.5% vs −10.3%, P = .0004). Efficacy according to success and improvement per Investigator's Global Assessment score and lesional percentage body surface area reached statistical significance based on central reader but not in-person assessments. Skin and subcutaneous tissue disorders were common all-causality treatment-emergent adverse events with crisaborole. Small sample size and short treatment duration were key limitations. In-person assessment was not conducted by dermatologists. Crisaborole improved signs and symptoms of SD and was well tolerated. Central reader assessment represents a promising approach for siteless clinical research.
Reference:
Jonathan I. Silverberg, Robert S. Kirsner, David J. Margolis, Michael Tharp, Daniela E. Myers, Karen Annis, Daniela Graham, Chuanbo Zang, Bonnie L. Vlahos, Paul Sanders,Efficacy and safety of crisaborole ointment, 2%, in participants aged ≥45 years with stasis dermatitis: Results from a fully decentralized, randomized, proof-of-concept phase 2a study,Journal of the American Academy of Dermatology,2024, ISSN 0190-9622. https://doi.org/10.1016/j.jaad.2023.12.048.(https://www.sciencedirect.com/science/article/pii/S0190962224000525)
Crisaborole, stasis dermatitis, Jonathan I. Silverberg, Robert S. Kirsner, David J. Margolis, Michael Tharp, Daniela E. Myers, Karen Annis, Daniela Graham, Chuanbo Zang, Bonnie L. Vlahos, Paul Sanders, crisaborole ointment, 2%, chronic venous insufficiency; crisaborole; decentralized study; phosphodiesterase-4 inhibitor; pruritus; stasis dermatitis; topical ointment; total sign score
Dr. Shravani Dali has completed her BDS from Pravara institute of medical sciences, loni. Following which she extensively worked in the healthcare sector for 2+ years. She has been actively involved in writing blogs in field of health and wellness. Currently she is pursuing her Masters of public health-health administration from Tata institute of social sciences. She can be contacted at editorial@medicaldialogues.in.


