- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Povorcitinib Shows Positive Outcomes For Hidradenitis Suppurativa in phase 2 trial
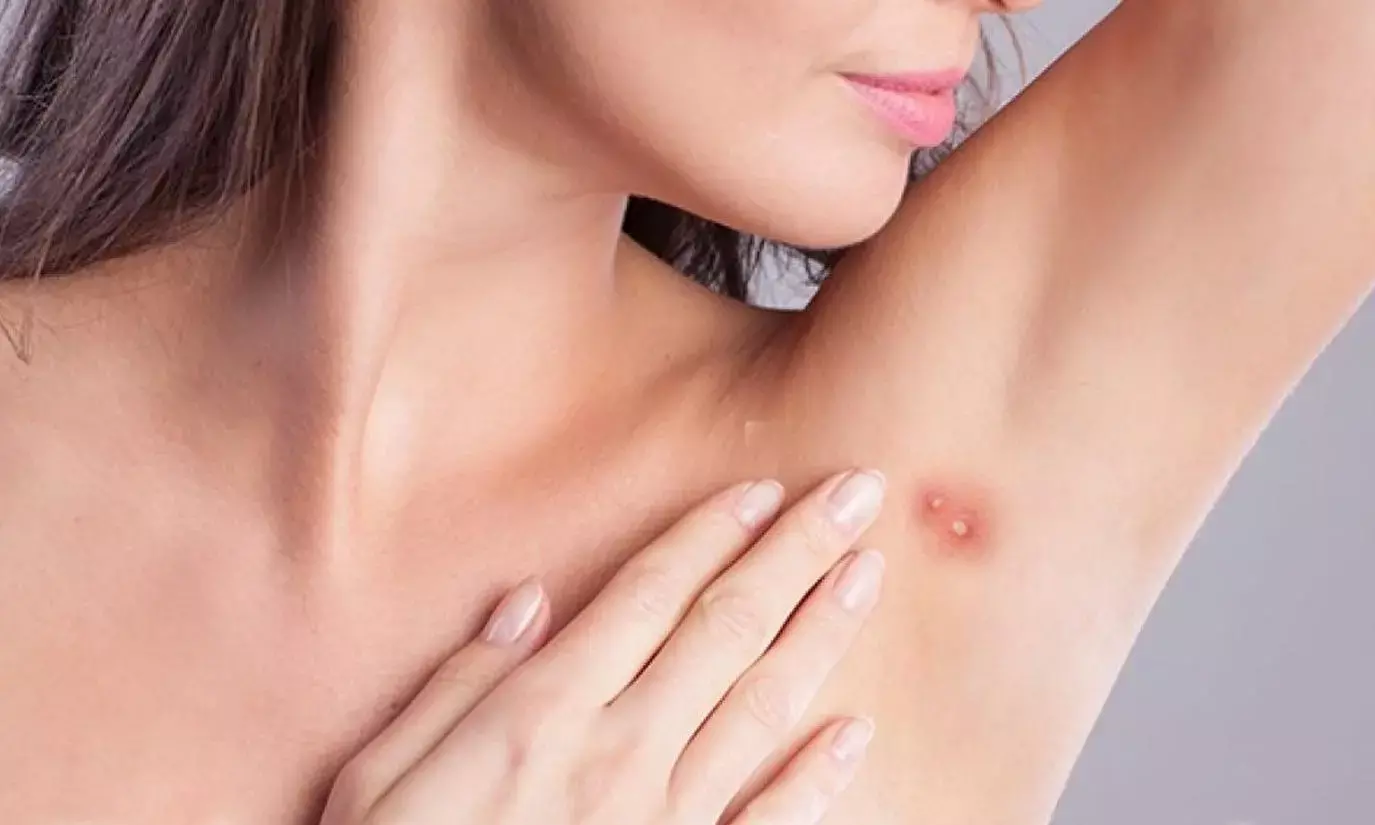
A recent study published in the Journal of the American Academy of Dermatology found the potential benefits of povorcitinib, a selective oral Janus kinase 1 (JAK1) inhibitor for treating Hidradenitis Suppurativa (HS), a chronic skin condition marked by painful nodules and abscesses.
This placebo-controlled phase 2 study involved a total of 209 patients, povorcitinib exhibited significant promise in reducing both abscess and inflammatory nodule (AN) counts over a 16-week period. Patients were further randomized to receive varying doses of povorcitinib—15 mg, 45 mg, or 75 mg—or a placebo.
The findings revealed a notable decrease in AN counts compared to the placebo group. The least squares mean change from baseline in AN count for the povorcitinib groups were as follows: 15 mg (−5.2), 45 mg (−6.9), and 75 mg (−6.3), whereas the placebo group showed a change of −2.5. The results suggest that povorcitinib may effectively alleviate HS-associated inflammation.
Additionally, the study evaluated the percentage of patients achieving Hidradenitis Suppurativa Clinical Response (HiSCR) at Week 16. Povorcitinib-treated patients demonstrated a higher likelihood of achieving HiSCR compared to the placebo group: 15 mg (48.1%), 45 mg (44.2%), and 75 mg (45.3%) versus placebo (28.8%).
Importantly, this study did not find evidence of an increased incidence of adverse events among the povorcitinib-treated patients, with 60.0% and 65.4% of povorcitinib and placebo groups reporting adverse events, respectively.
While the results present an encouraging step forward in the quest for HS treatments, it is crucial to note the study's limitations, including mildly imbalanced baseline lesion counts between groups.
The potential of povorcitinib in alleviating HS symptoms marks a significant development in dermatological research. However, future research, including well-structured, large-scale clinical trials, will be necessary to solidify its efficacy and safety profile for widespread use.
Source:
Kirby, J. S., Okun, M. M., Alavi, A., Bechara, F. G., Zouboulis, C. C., Brown, K., Santos, L. L., Wang, A., Bibeau, K. B., Kimball, A. B., & Porter, M. L. (2023). Efficacy and safety of the oral Janus kinase 1 inhibitor povorcitinib (INCB054707) in patients with hidradenitis suppurativa in a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study. In Journal of the American Academy of Dermatology. Elsevier BV. https://doi.org/10.1016/j.jaad.2023.10.034
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


