- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Rituximab superior to Mycophenolate mofetil for complete remission of Pemphigus Vulgaris: Study
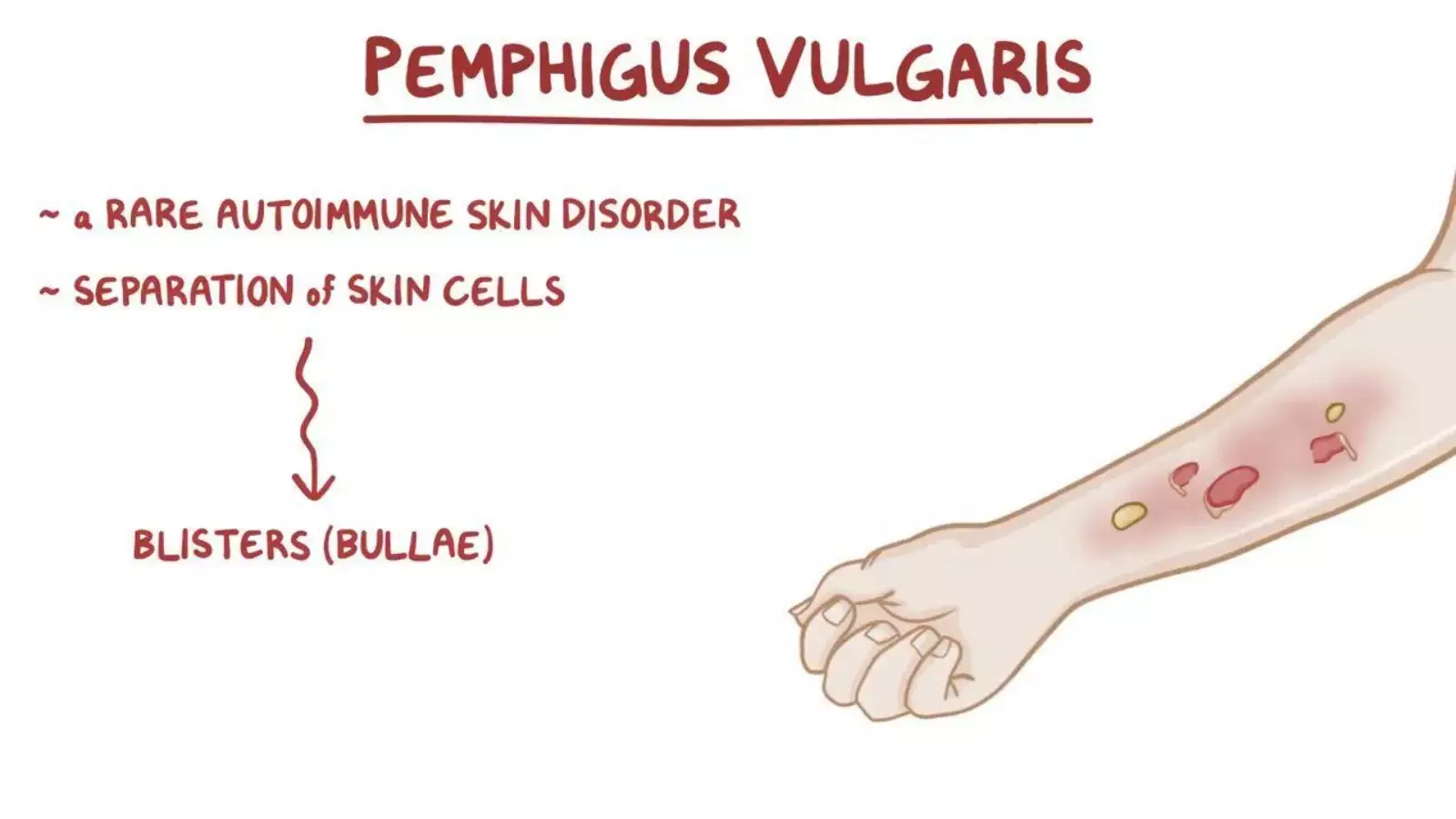
Rituximab showed better outcomes than mycophenolate mofetil in producing continued complete remission at 52 weeks in patients suffering from pemphigus vulgaris, according to a study published in The New England Journal of Medicine.
Pemphigus vulgaris is a rare and often life-threatening autoimmune disease. It is characterized by blistering and erosion of the skin and mucous membranes. It specifically occurs in either middle-aged or older people. The primary lesion of pemphigus vulgaris is a soft blister filled with clear fluid that appears on healthy or irritated skin. Rituximab and mycophenolate mofetil have been actively used to cure pemphigus vulgaris, however, their individual efficacies have never been thoroughly compared.
A study was conducted by a group of researchers from the U.K., to adequately compare Rituximab and Mycophenolate mofetil used in the treatment of pemphigus vulgaris in clinical trials.
The researchers conducted a randomized, controlled trial, wherein they categorized a total of 135 moderate-severe patients suffering from pemphigus vulgaris into two groups and were administered either intravenous rituximab (67 patients with 1000 mg on days 1, 15, 168, and 182) or oral mycophenolate mofetil (68 patients with 2 g per day) in a 1:1 ratio. Additionally, both the groups were also administered an oral glucocorticoid was also administered on the same tapering schedule.
Ø The primary endpoint was sustained complete remission at week 52, defined as the healing of lesions with no new active lesions, as reflected by a Pemphigus Disease Area Index (PDAI) activity a score of 0 (on a scale of 0 to 250, with higher scores indicating greater disease severity), for at least 16 weeks without the use of glucocorticoids.
Ø While the secondary end points were the total dose of glucocorticoids, the number of disease flares, and the change from baseline in the score on the Dermatology Life Quality Index (DLQI; scores range from 0 to 30, with higher scores indicating greater impairment).
The findings were as follows:
Ø The primary outcome was assessed in the modified intention-to-treat population: 62 patients in the rituximab group and 63 in the mycophenolate mofetil group.
Ø The median PDAI activity score was better in the group that received Mycophenolate mofetil (18.3) as compared to the Rituximab group (22.7).
Ø At week 52, in a total of 25 patients, continued complete remission was seen more in the rituximab group (40%) as compared to the mycophenolate mofetil group (10%) (difference, 31 percentage points; 95% confidence interval [CI], 15 to 45; P<0.001).
Ø The average total glucocorticoid dosage during the 52-week treatment period was more in the mycophenolate group (5140 mg) than the rituximab group (3545 mg) (difference, −1595 mg; 95% CI, −2838 to −353; P<0.001).
Ø Also, the disease flares were more in the mycophenolate group (44) as compared to the rituximab group (6) (adjusted rate ratio, 0.12; 95% CI, 0.05 to 0.29; P<0.001).
Ø The mean change in DLQI the score was −8.87 points and −6.00 points, respectively (difference, −2.87 points; 95% CI, −4.58 to −1.17; P=0.001).
Ø Serious adverse events occurred more frequently in the rituximab group (22%) than the mycophenolate mofetil group (15%).
The authors concluded that though Rituximab was more favorable than mycophenolate mofetil in producing sustained complete remission at 52 weeks in patients with pemphigus vulgaris, it caused more serious adverse events. Hence further evaluation to investigate the comparative efficacy and safety of rituximab and mycophenolate mofetil beyond 52 weeks of treatment is required.
Reference
A study titled, "Rituximab versus Mycophenolate Mofetil in Patients with Pemphigus Vulgaris" by Werth V et. al published in The New England Journal of Medicine.
DOI: 10.1056/NEJMoa2028564
Dr. Shravani Dali has completed her BDS from Pravara institute of medical sciences, loni. Following which she extensively worked in the healthcare sector for 2+ years. She has been actively involved in writing blogs in field of health and wellness. Currently she is pursuing her Masters of public health-health administration from Tata institute of social sciences. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


