- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA approves antiplatelet drug Kengreal based on Champion Phoenix Study
The Medicines Company Announced FDA Approval of KENGREAL™ (cangrelor) as an Adjunct to Percutaneous Coronary Intervention (PCI) for Reducing Thrombotic Events
Patients undergoing Coronary intervention, a common heart procedure in India and US where a stent is implanted to prevent artery blockage, is up for some good news to safeguard their health interests. Blood clotting, through the medically termed procedure percunateous coronary intervention (PCI), can be prevented through Cangrelor (Kengreal).
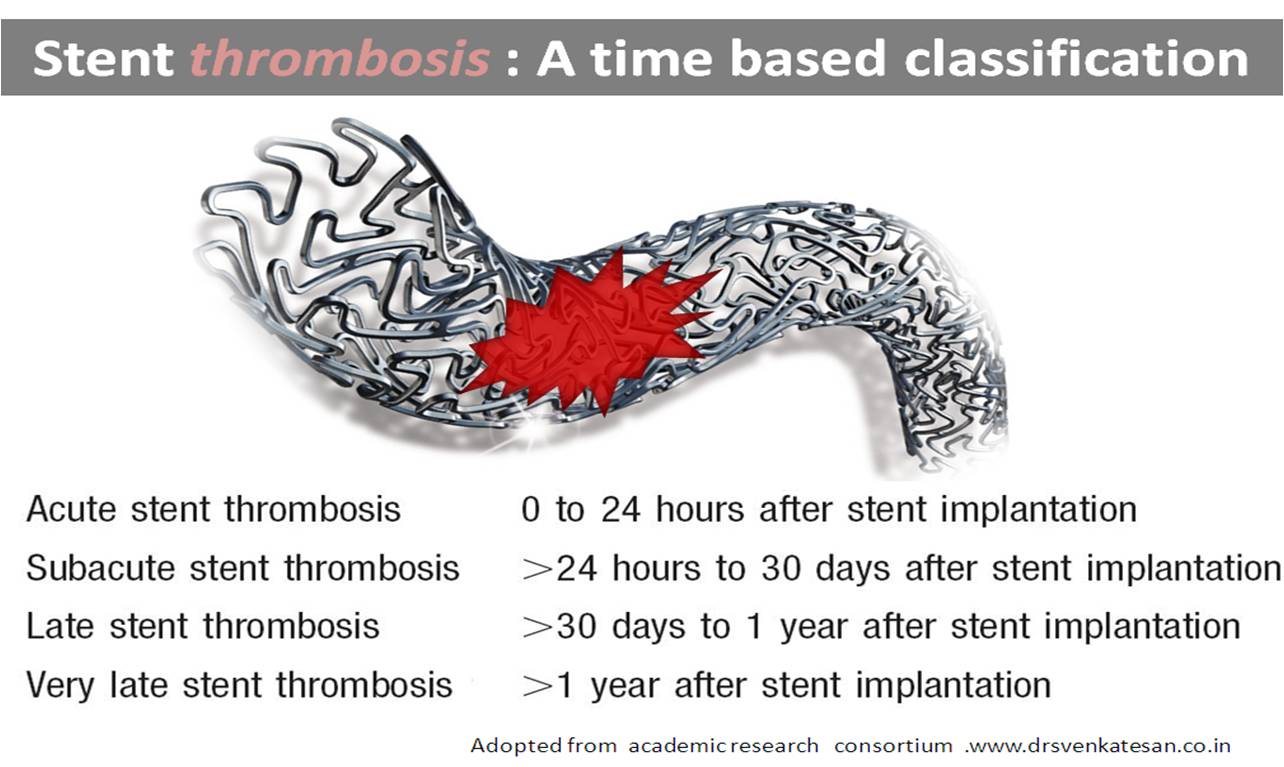
The FDA has approved the drug to reduce the chances of harmful blood clotting, which exposes the patient to increased risk on a heart stroke. By preventing platelets from accumulating, the drug reduces the chance of stent choking as well ( Stent thrombosis). However, in a clinical trial conducted in the US, the most extreme risk of the drug is excessive bleeding, including life threatening bleeding.
The FDA approval is based on data from the large-scale CHAMPION-PHOENIX STUDY, an 11,145-patient randomized trial comparing intravenous cangrelor against clopidogrel. The study, which was led by Dr Deepak Bhatt (Brigham and Women's Hospital, MA), included patients undergoing PCI for stable angina or acute coronary syndromes, including ST-segment elevation MI.
The Medicines Company manufacturer of Kengreal sponsored CHAMPION-PHOENIX.
Meghna A Singhania is the founder and Editor-in-Chief at Medical Dialogues. An Economics graduate from Delhi University and a post graduate from London School of Economics and Political Science, her key research interest lies in health economics, and policy making in health and medical sector in the country. She is a member of the Association of Healthcare Journalists. She can be contacted at meghna@medicaldialogues.in. Contact no. 011-43720751
Next Story


