- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Antiviral tecovirimat safe and effective for treating monkeypox infection: JAMA
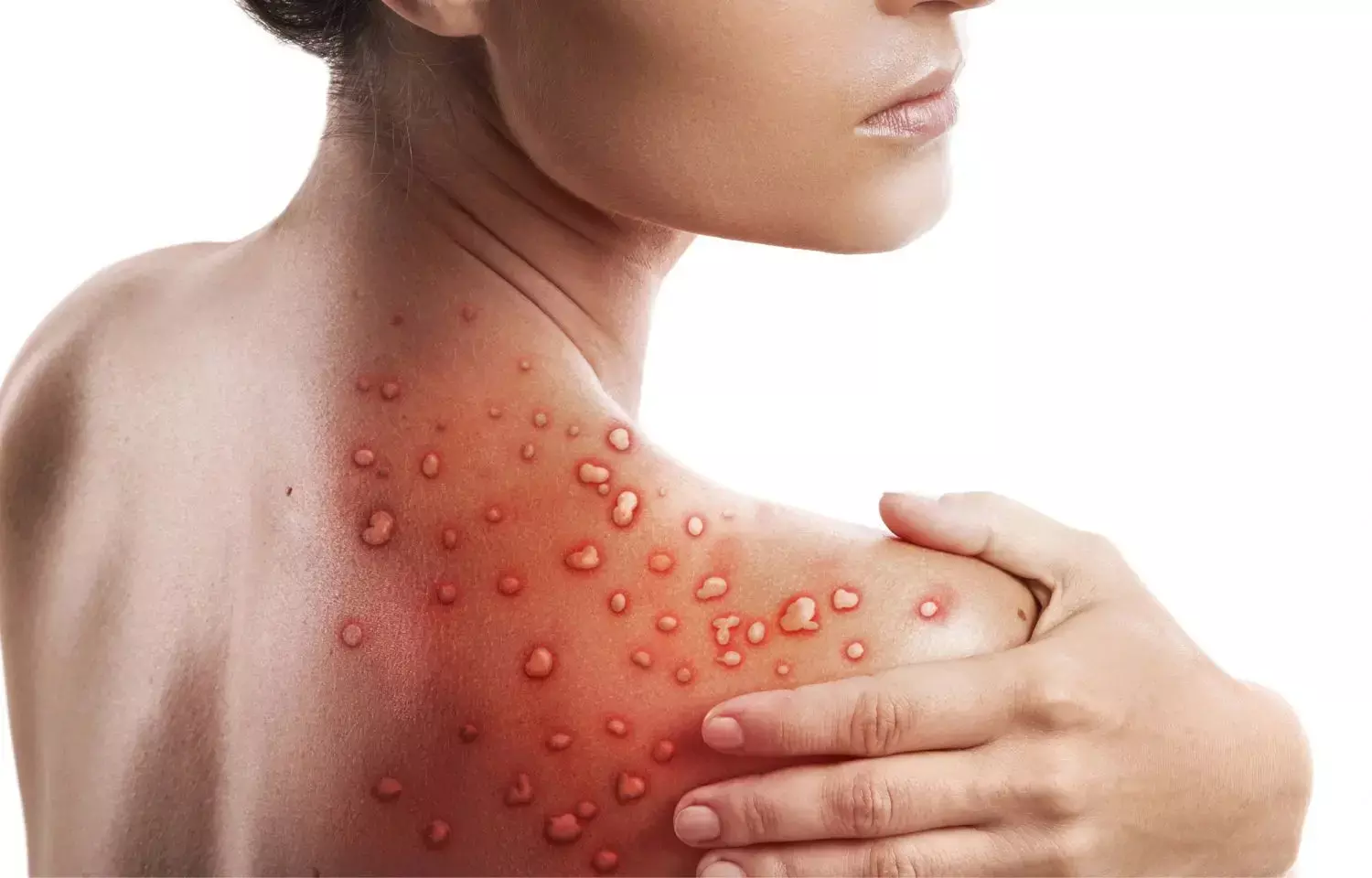
California: Compassionate use of oral tecovirimat is well-tolerated for the treatment of monkeypox infection with minimal side effects, researchers state in a research letter published in the Journal of the American Medical Association (JAMA). It further stated that adverse effects, however, could not always be differentiated from symptoms related to the infection.
Monkeypox is a zoonotic orthopoxvirus that belongs to the same genus as variola -- the causative agent of smallpox. A recent global outbreak has resulted in more than 39 000 cases reported as of August 18, 2022. Monkeypox is typically self-limited and its symptoms last generally between 2 and 4 weeks in prior outbreaks. In a recent study, hospitalization was needed in 13% of patients indicating the need for effective therapy.
Tecovirimat is an antiviral that is shown to inhibit p37, a protein involved in the release of the enveloped virus, dissemination, and viral virulence. It is shown to have activity both against smallpox and monkeypox.
Against the above background, Angel N. Desai, Division of Infectious Diseases, University of California Davis Medical Center, Sacramento, California, and colleagues aimed to assess adverse events and clinical resolution of systemic symptoms and lesions in an uncontrolled cohort study of patients with monkeypox who were treated with tecovirimat on a compassionate use basis.
The researchers included patients after laboratory confirmation of orthopoxvirus infection from skin lesions by polymerase chain reaction. Tecovirimat treatment for adult patients was based on weight and was administered every 8 or 12 hours, and was taken within 30 minutes of a meal containing moderate to high-fat content for improved bioavailability.
therapy duration was 14 days but could be extended based on the clinical status of the patient. Collection of clinical data was done at initial in-person evaluation for treatment and by in-person or telephone interview on day 7 and day 21 following initiation of therapy. Written informed consent was given by all patients.
The study led to the following findings:
- As of August 13, 2022, 25 patients with confirmed monkeypox infection had completed a course of tecovirimat therapy. All patients were self-reported male and the median age was 40.7 years.
- Nine patients had HIV, 1 patient had received the smallpox vaccine more than 25 years prior, and 4 received 1 dose of JYNNEOS vaccination after symptom onset.
- At the time of treatment, systemic symptoms, lesions, or both were present for a mean of 12 days.
- Systemic symptoms included fever in 76% of patients, headache in 32%, fatigue in 28%, sore throat in 20%, chill in 20%, backache in 12%, myalgia in 8%, nausea in 4%, and diarrhea in 4%.
- Almost all patients (92%) had genital and/or perianal lesions, and 52% had fewer than 10 lesions over their entire body.
- All patients had pain associated with lesions.
- One patient received 21 days of therapy while the remainder were treated for 14 days.
- Complete resolution of lesions was reported in 40% of patients on day 7 of therapy, while 92% had resolution of lesions and pain by day 21.
- Treatment with tecovirimat was generally well tolerated with no patient discontinuing therapy.
- The most frequently reported adverse events on day 7 of therapy included the following: fatigue in 28% of patients, headache in 20%, nausea in 16%, itching in 8%, and diarrhea in 8%.
"The conclusions of antiviral efficacy pertaining to the duration of symptoms or severity were limited as no control group was included," the researchers wrote. "Time from symptom onset to presentation was variable among patients, and conclusions related to antiviral use vs natural evolution of disease should be made with caution."
Reference:
Desai AN, Thompson GR, Neumeister SM, Arutyunova AM, Trigg K, Cohen SH. Compassionate Use of Tecovirimat for the Treatment of Monkeypox Infection. JAMA. Published online August 22, 2022. doi:10.1001/jama.2022.15336
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


