- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
WHO AND EMA expert panel confirms safety of AstraZeneca's vaccine after clotting reports
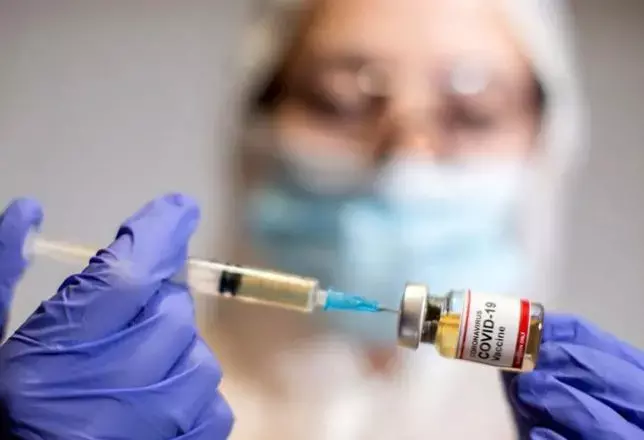
In a recent update, the World Health Organization (WHO) and the European Medicines Agency (EMA), combinedly has put forward a declaration that benefits of preventing hospitalization and death from COVID-19 outweigh the possible risks of thromboembolic events and thrombocytopenia, which are clearly rare, after receiving AstraZeneca's COVID-19 vaccine.
The vaccine had been put on hold after 7 cases of blood clots in multiple blood vessels (disseminated intravascular coagulation, DIC) and 18 cases of CVST were reported in patients receiving the doses, urging an immediate investigation on the safety of the vaccine.
The Committee's experts looked in extreme detail at records of DIC and CVST reported from Member States, 9 of which resulted in death. Most of these occurred in people under 55 and the majority were women. "Because these events are rare, and COVID-19 itself often causes blood clotting disorders in patients, it is difficult to estimate a background rate for these events in people who have not had the vaccine."the panel opined.
Moving a step further to ensure safety , the expert panel has advised that , though rare,if symptoms suggestive of clotting problems occur patients should seek immediate medical attention and inform healthcare professionals of their recent vaccination. Also,Steps are already being taken to update the product details for the vaccine to include more information on these risks.
Key highlights from the declaration has been provided below.
The Committee confirmed that:
- The benefits of the vaccine in combating the still widespread threat of COVID-19 (which itself results in clotting problems and may be fatal) continue to outweigh the risk of side effects;
- The vaccine is not associated with an increase in the overall risk of blood clots (thromboembolic events) in those who receive it;
- There is no evidence of a problem related to specific batches of the vaccine or to particular manufacturing sites;
- However , the vaccine may be associated with very rare cases of blood clots associated with thrombocytopenia, i.e. low levels of blood platelets (elements in the blood that help it to clot) with or without bleeding, including rare cases of clots in the vessels draining blood from the brain (CVST).
For the full article follow the link: Press briefing on the conclusion of the investigation of COVID-19 Vaccine AstraZeneca and thromboembolic events by the Pharmacovigilance Risk Assessment Committee (PRAC) (18/03/2021)
Dr Satabdi Saha (BDS, MDS) is a practicing pediatric dentist with a keen interest in new medical researches and updates. She has completed her BDS from North Bengal Dental College ,Darjeeling. Then she went on to secure an ALL INDIA NEET PG rank and completed her MDS from the first dental college in the country – Dr R. Ahmed Dental College and Hospital. She is currently attached to The Marwari Relief Society Hospital as a consultant along with private practice of 2 years. She has published scientific papers in national and international journals. Her strong passion of sharing knowledge with the medical fraternity has motivated her to be a part of Medical Dialogues.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


