- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
DCGI suspends permission to Entod Pharma to manufacture, market PresVu, What next for the Company?
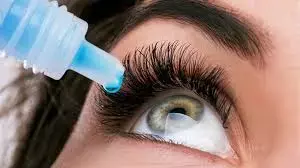
New Delhi: In response to the various alleged misleading claims made by Entod Pharma in respect to its Opthalmologic product, Drugs Controller General of India (DCGI) has suspended permission given to it for the manufacturing, and marketing of Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% w/v for the treatment of Presbyopia in adults.
The permission is suspended till further order under-the provisions of Rule 84 of the New Drugs and Clinics trials Rules, 2019 of the Drugs and Cosmetics Act, 1940
The Drug regulator has said that the Company had tried to justify the claims for the product for which no approval was granted.
The Directorate had granted permission on August 20 to the Company for manufacture and marketing of Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% w/v for the treatment of Presbyopia in adults. Following this, a press conference was organized by the company where several misleading claims were allegedly made by the company officials which finally led to several media articles spreading misleading claims and information in the public.
Medical Dialogues team had done a fact-check story in this matter, which can be accessed on the link below
Read Here: Fact Check: Can Entod Pharma's PresVu get rid of reading glasses?
DCGI Response: In this regard, Drug regulator informed the company that the Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% w/v has not been approved for any such claim that it is designed to reduce the need for reading glasses.
2 ) Claim 2: Non-invasive option that can enhance near vision without the need for reading glasses
Company Submission to DCGI: In response to the another claim "this eye drop offers a non-invasive option that can enhance near vision without the need for reading glasses' the Company stated that in the clinical trial conducted, subjects did not wear glasses to participate etc.
Following the order, ENTOD CEO Masurkar said in a statement that the company will challenge this suspension in the court.
"We at ENTOD Pharmaceuticals hereby declare that we have not made any unethical or false presentation of facts to the media or public when it comes to Presvu Eye Drops," he said. According to a PTI report, the CEO said that the Company has got suspension order with no reference to any specific violation of Drugs and Cosmetics Act for this action.
."We strongly desist this action against a proud Indian pharma company in the MSME sector company like Entod Pharmaceuticals which is purely research and innovation driven and attempts to bring new therapeutic options to the Indian market," he said.
"As a result, we have decided to challenge this suspension in the court of law to get justice. Our fight will not only allow innovative medicines to be available in India but also encourage other pharmaceutical entrepreneurs and companies in the MSME sector to continue the research drive in India without facing similar obstacles," Masurkar added.
Ruchika Sharma joined Medical Dialogue as an Correspondent for the Business Section in 2019. She covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She has completed her B.Com from Delhi University and then pursued postgraduation in M.Com. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


