- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
ASSIST II study evaluated Feasibility, Safety, and Efficacy of the OdonAssist Device for Assisted Vaginal Births
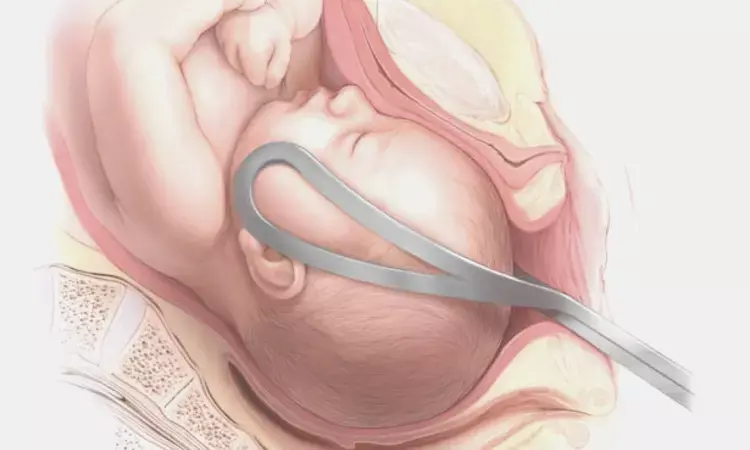
Recent study aimed to determine if the feasibility, safety, and efficacy of the OdonAssist device were sufficient to justify conducting a future randomized controlled trial for assisted vaginal births. The study involved 104 participants who received the OdonAssist intervention, as well as a nested cohort of participants who consented but did not receive the intervention.
Primary and Secondary Outcomes
The primary outcome was the proportion of births successfully assisted with the OdonAssist device. Secondary outcomes included clinical, patient-reported, operator-reported, device, and health care utilization measures, as well as maternal and neonatal outcomes. The study found that the success rate of the OdonAssist device was 66% (69/104), meeting the threshold for a future randomized controlled trial. There were no serious device-related maternal or neonatal adverse reactions, and potential advantages were observed for neonatal outcomes, with only 4% of the successful OdonAssist group experiencing any device-related adverse effects in the form of neonatal soft tissue bruising, compared to 20% in the unsuccessful OdonAssist group and 23% in the nested cohort.
Factors Affecting OdonAssist Success
Factors associated with higher OdonAssist success rates included shorter total second stage duration, lower station at birth, and more operator experience. Participants reported high birth perception scores, and all practitioners found the device easy to use.
Feasibility, Recruitment, and Future Trial Implications
The study demonstrated that recruitment to an interventional study of a new device for assisted vaginal birth is feasible, with 64% of eligible participants willing to participate. The results provide important data to inform the design of a future randomized controlled trial to compare the OdonAssist with current standard practice for assisted vaginal births.
Key Points
1. The study aimed to determine the feasibility, safety, and efficacy of the OdonAssist device for assisted vaginal births, involving 104 participants who received the intervention and a nested cohort who did not.
2. The primary outcome was the success rate of the OdonAssist device, which was 66%, meeting the threshold for a future randomized controlled trial. There were no serious device-related adverse reactions, and potential advantages were observed for neonatal outcomes.
3. Factors associated with higher OdonAssist success rates included shorter total second stage duration, lower station at birth, and more operator experience. Participants reported high birth perception scores, and practitioners found the device easy to use.
4. The study demonstrated that recruitment to an interventional study of a new device for assisted vaginal birth is feasible, with 64% of eligible participants willing to participate.
5. The results provide important data to inform the design of a future randomized controlled trial to compare the OdonAssist with current standard practice for assisted vaginal births.
6. Overall, the study suggests that the feasibility, safety, and efficacy of the OdonAssist device are sufficient to justify conducting a future randomized controlled trial for assisted vaginal births.
Reference -
Hotton EJ, Bale N, Rose C, White P, Wade J, Mottet N, Loose AJ, Elhodaiby M, Lenguerrand E, Draycott TJ, Crofts JF; ASSIST II Study Group. The OdonAssist inflatable device for assisted vaginal birth-the ASSIST II study (United Kingdom). Am J Obstet Gynecol. 2024 Mar;230(3S):S932-S946.e3. doi: 10.1016/j.ajog.2023.05.018. Epub 2023 Jul 30. PMID: 38462264.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


