- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
CSTTA may prevent brolucizumab related intraocular inflammation in nAMD
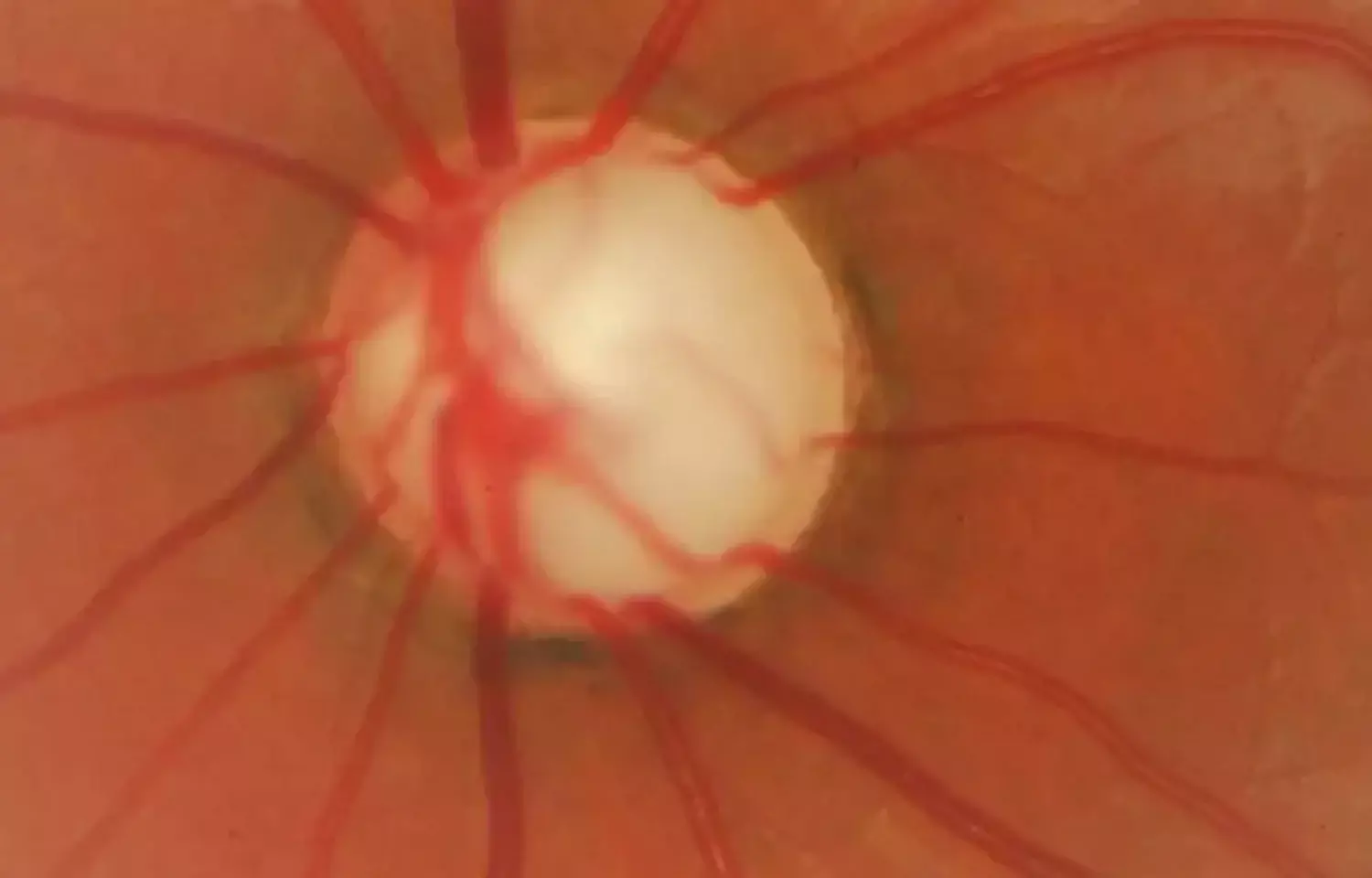
Concomitant use of sub-tenon's capsule triamcinolone acetonide with brolucizumab may prevent intraocular inflammation according to a recent study published in Graefe's Archive for Clinical and Experimental Ophthalmology.
A study was conducted to investigate the efficacy of sub-Tenon's capsule triamcinolone acetonide (STTA) injections for preventing the development of intraocular inflammation (IOI) related to intravitreal injection (IVI) of brolucizumab for neovascular age-related macular degeneration (nAMD).
Consecutive patients with nAMD treated with brolucizumab IVIs were studied retrospectively. All eyes treated with brolucizumab in the clinic were switched from another anti-vascular endothelial growth factor agent. After the fourth case of IOI related to brolucizumab IVI, all eyes treated with brolucizumab received an STTA injection. The patients were divided into two groups: brolucizumab alone and brolucizumab combined with an STTA injection.
Results:
- Forty-four eyes (44 patients) treated with at least one brolucizumab IVI have studied: 14 eyes received brolucizumab IVI alone and 30 eyes received the combination therapy.
- IOI related to brolucizumab IVIs developed in four (28.6%) of 14 eyes in the brolucizumab group; IOI was severe in one eye, moderate in two eyes, and mild in one eye according to the HAWK and HARRIER trial definition; IOI did not develop in the 30 eyes that received combination therapy, the difference of which reached significance (p = 0.012).
- Regarding combination therapy, the intraocular pressure in three (10%) eyes increased to 22 to about 26 mmHg after the STTA injection and returned to the normal range within 2 months without medication; no cataracts developed during this short mean follow-up period of 7.1 ± 0.4 months.
Thus, the results indicated the possible preventative effect of an STTA injection on the development of brolucizumab-associated IOI.
Reference:
Hikichi, T. Sub-Tenon's capsule triamcinolone acetonide injection to prevent brolucizumab-associated intraocular inflammation. Graefes Arch Clin Exp Ophthalmol 260, 2529–2535 (2022). https://doi.org/10.1007/s00417-022-05611-y
Keywords:
Concomitant use, STTA, brolucizumab, prevent, intraocular, inflammation, Graefe's Archive for Clinical and Experimental Ophthalmology, Hikichi, T., triamcinolone acetonide, Sub-Tenon's capsule triamcinolone acetonide injection, Anti-vascular endothelial growth factor therapy, Intraocular inflammation, Neovascular age-related macular degeneration
Dr. Shravani Dali has completed her BDS from Pravara institute of medical sciences, loni. Following which she extensively worked in the healthcare sector for 2+ years. She has been actively involved in writing blogs in field of health and wellness. Currently she is pursuing her Masters of public health-health administration from Tata institute of social sciences. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


