- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Intravitreal Faricimab has Promising Outcomes for Diabetic Macular Edema Patients
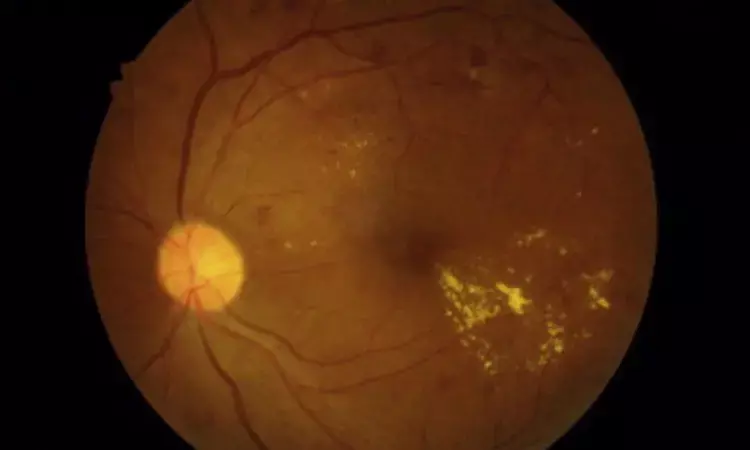
In a significant breakthrough, a recent study has uncovered encouraging results with significantly improved functional and anatomical outcomes at 12 months for patients suffering from diabetic macular edema (DME) who have shown resistance to the commonly prescribed intravitreal aflibercept (IVA). The recent retrospective interventional case series has shed light on the potential benefits of intravitreal faricimab (IVF) in treating diabetic macular edema (DME) which has proven resistant to intravitreal aflibercept (IVA).
The study results were published in the Journal 'Clinical Ophthalmology' on August 16, 2023.
Approved by the FDA, Faricimab has gained significant attention with successful outcomes in diabetic macular edema (DME) who were earlier treated with intravitreal aflibercept. Previous trials were short-term trials conducted for a 4-month duration, hence research team led by Ryan B Rush from Texas conducted a retrospective interventional case series study to assess the 12-month outcomes of intravitreal faricimab (IVF) in treatment-resistant diabetic macular edema (DME) recalcitrant to intravitreal aflibercept (IVA).
Subjects who met the specific criteria of a history of at least eight IVA injections in the preceding year, a minimum of four IVA injections within the previous six months, a central macular thickness (CMT) measurement of 320 microns or more using optical coherence tomography (OCT), and observable edema on OCT scans were taken up for the study. Throughout the study, subjects followed a treat-and-extend (TAE) protocol and were tracked for 12 months from this critical baseline visit. A total of 51 eyes of 51 subjects were analyzed.
Findings:
- Remarkably 39.2% (20/51) of patients who reached a treatment interval of ≥8 weeks had a fluid-free macula on OCT at 12 months.
- There was a significant reduction in the CMT on OCT from 400.2 (385.3–415.3) microns at baseline to 340.6 (324.3–356.9) microns at 12 months (p<0.01).
- About 21.6% (11/51) of patients improved ≥3 lines of Snellen visual acuity at 12 months.
- The overall visual acuity of the entire study population exhibited significant enhancement, shifting from an average of 0.60 logMAR (Snellen 20/80) at baseline to 0.47 logMAR (Snellen 20/59) at the 12-month mark (p<0.01).
These findings bear immense promise for a subset of DME patients who have shown resistance to aflibercept. By transitioning to IVF treatment and adopting a TAE protocol, individuals experiencing this form of resistance may look forward to extended treatment intervals and the potential for improved functional and anatomical outcomes over the course of 12 months. This study's revelations provide a ray of hope and new treatment avenues for those grappling with the challenges of DME, offering the prospect of improved vision and overall eye health.
Further reading: Rush RB. One Year Results of Faricimab for Aflibercept-Resistant Diabetic Macular Edema. Clin Ophthalmol. 2023 Aug 16;17:2397-2403. doi: 10.2147/OPTH.S424314. PMID: 37605765; PMCID: PMC10440101.
BDS, MDS
Dr.Niharika Harsha B (BDS,MDS) completed her BDS from Govt Dental College, Hyderabad and MDS from Dr.NTR University of health sciences(Now Kaloji Rao University). She has 4 years of private dental practice and worked for 2 years as Consultant Oral Radiologist at a Dental Imaging Centre in Hyderabad. She worked as Research Assistant and scientific writer in the development of Oral Anti cancer screening device with her seniors. She has a deep intriguing wish in writing highly engaging, captivating and informative medical content for a wider audience. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751



